 |
|
|
|
Molecular recognition is seen with the bare eye |
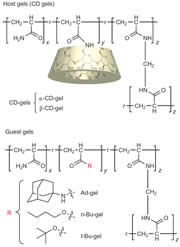
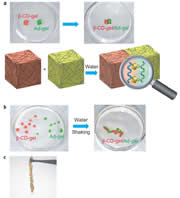
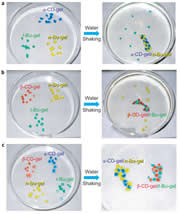
We find that two gels join together strongly only by contacting the host (cyclodextrin, CD)-bearing gel with the guest-bearing gel. Moreover, adhesion and dissociation of the gels are controllable by exogenous stimuli, such as light, solvent composition, temperature, pH, and redox. The adhesion of gels can be accomplished by not only by molecule recognition but also by a coordinate bond, a hydrogen bond, and a covalent bond.
Macroscopic self-assembly through molecular recognition
We prepared β-cyclodextrin gel (β-CD-gel) and adamantine gel (Ad-gel).β-CD-gel was found to bind Ad-gel strongly through molecular recognition. In addition, a mixture of pieces of α-CD-gel, β-CD-gel, n-Bu-gel and t-Bu-gel exhibited excellent fidelity only by mixing and shaking in water; α-CD-gel specifically adhered to n-Bu-gel, and β-CD-gel selectively adhered to t-Bu-gel to form macroscopic self-assemblies.
Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H., Nature Chem. 2011, 3, 34-37.
Yamaguchi, H.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A., Macromolecules 2011, 44 (8), 2395-2399.
Zheng, Y.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A, Langmuir 2011, 27(22), 13790-13795.
Nature (Highlight), Chemistry World, etc.
 |
|
 |
|
 |