触媒1
(1) 人工ポルフィリンー蛋白質錯体で最も効率の良い水素発生システムの構築 (2006)
Photoinduced Hydrogen Generation
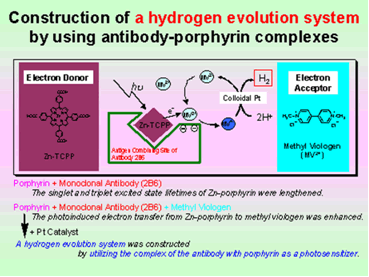
Hydrogen Evolution System Using Antibody-Porphyrin-Complex as Photosensitizer
One of the complexes between monoclonal antibody 2B6 for porphyrin and zinc porphyrin (Zn-porphyrin) was used to construct an energy conversion system. The antibody 2B6 could bind Zn-porphyrin with a dissociation constant of 2.1 x 10-8 M, estimated by ELISA. When antibody 2B6 was added to an aqueous solution of Zn-porphyrin, the lifetime of its singlet exited state was lengthened from 1.7 to 2.1 ns. The triplet state lifetime of Zn-porphyrin was also lengthened from 0.5 to 1.2 ms.
Continuous light irradiation of Zn-porphyrin was performed in the presence of the antibody and MV. The color of the solution turned blue and a product with a maximum absorbance at 602 nm appeared. Methyl viologen cationic radical was obtained by the photoinduced electron transfer from porphyrin in the binding site of the antibody to MV. The half-life of methyl viologen cationic radical was over 15 min. No color changes were observed without antibodies. The antibody was found to catalyze the electron transfer to give a stable cationic radical. We found that the cationic radical obtained in the antibody–porphyrin complex system could be utilized for producing chemical energy, hydrogen. The antibody–porphyrin complex, MV, and colloidal Pt were equipped for the construction of a hydrogen evolution system. An increase of the concentration of hydrogen was observed in the solution of the antibody–porphyrin complex. The electron transfer could take place efficiently from porphyrin in the antibody binding site to MV outside of the binding pocket.
(2) 時として天然酵素に勝る触媒活性をもつ抗体ーポルフィリン錯体 (2004)
Peroxidase Activity of Metalloporphyrin-Antibody Complex
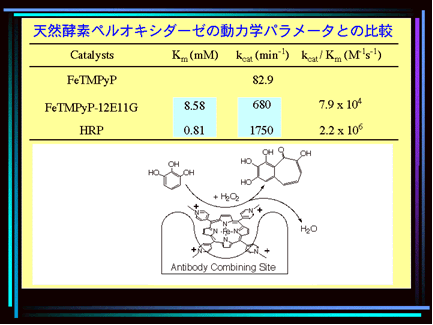
An interesting development is the use of natural macromolecules as carriers for artificial catalysts. One example is the cationic meso-tetrakis(4-N-methylpyridyl)porphyrin and its analogues, which are known to bind to DNA. H. Yamaguchi and A. Harada (Osaka, Japan) bound the FeII complex of this porphyrin to the monoclonal antibody 12E11G. This macromolecular metal complex displays remarkable activity in the oxidation of catechol, guajacol, and pyrogallol in the presence of H2O2, and exhibits a peroxidase activity that lies in the same order as that of horseradish peroxidase while showing a better stability than the natural system.
Reported by Dieter Wohrle in Angew. Chem. Int. Ed., 2005, 44, 7500-7502.
ページのTOPへ


