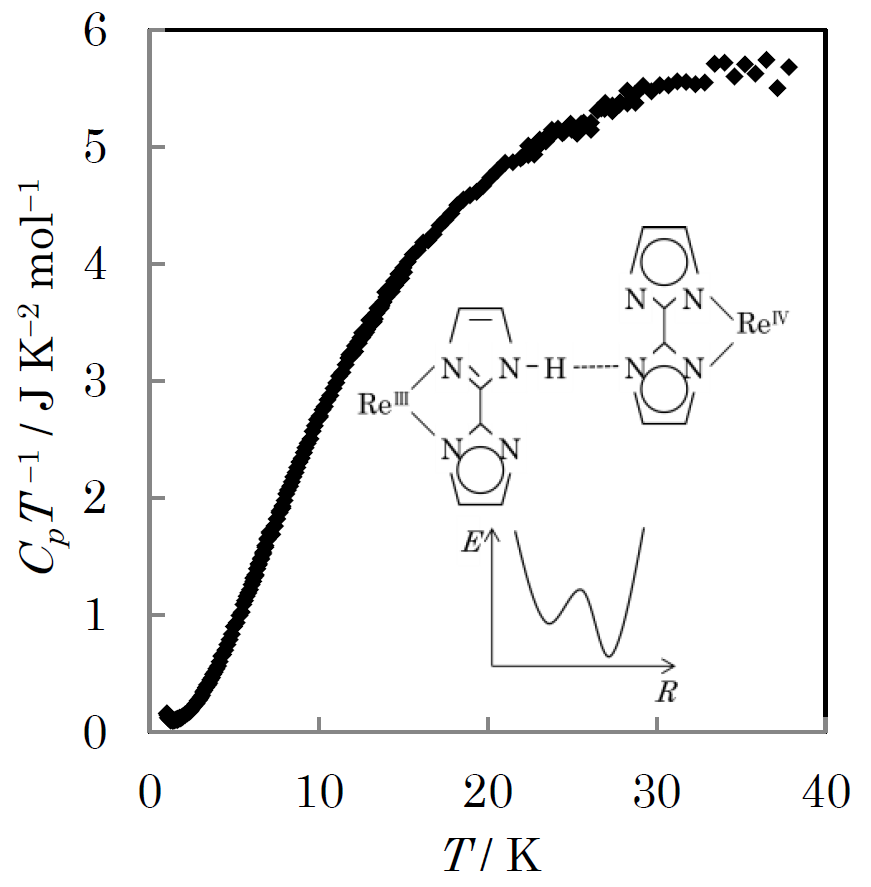
Fig.1. Temperature dependence of heat capacity of [ReIIICl2(PPr3)2 (Hbim)][ReIVCl2(PPr3)2(bim)].
The compound of [ReIIICl2(PPr3)2 (Hbim)][ReIVCl2(PPr3)2(bim)] shows the proton transfer in the N-H…N hydrogen bond. This proton transfer is coupled with electron transfer between ReIIIand ReIVat higher temperature. However, the ESR measurement suggested a possibility of phase transition related to proton localization around 20 K. The structural phase transition occurs between 213 K and 233 K produces a change of the single energy potential of hydrogen bonding and makes an asymmetric structure. In this study, We have performed heat capacity measurement of [ReIIICl2(PPr3)2 (Hbim)][ReIVCl2(PPr3)2(bim)] in order to investigate the proton localization nature at low temperature. We observed a broad thermal anomaly around 5 K which is slightly lower than the suggested temperature. This anomaly is a Schottky type anomaly due to a asymmetric double minimum potential.

Fig.1. Temperature dependence of heat capacity of [ReIIICl2(PPr3)2 (Hbim)][ReIVCl2(PPr3)2(bim)].
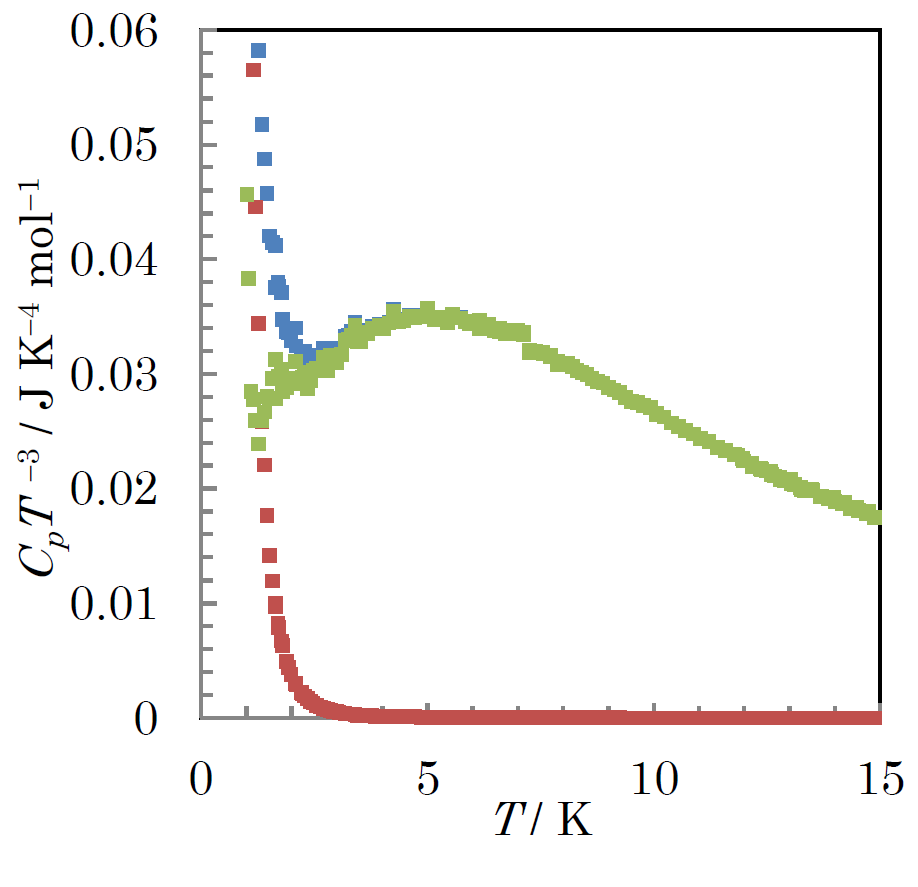
Fig.2.(Color online)Blue plot shows temperature dependence of total heat capacity of [ReIIICl2(PPr3)2 (Hbim)][ReIVCl2(PPr3)2(bim)] below 15 K in a CpT−3 vs T plot. Red plot indicates the Schottky type magnetic heat capacity. Green plot indicates heat capacity without the Schottky type magnetic heat capacity.
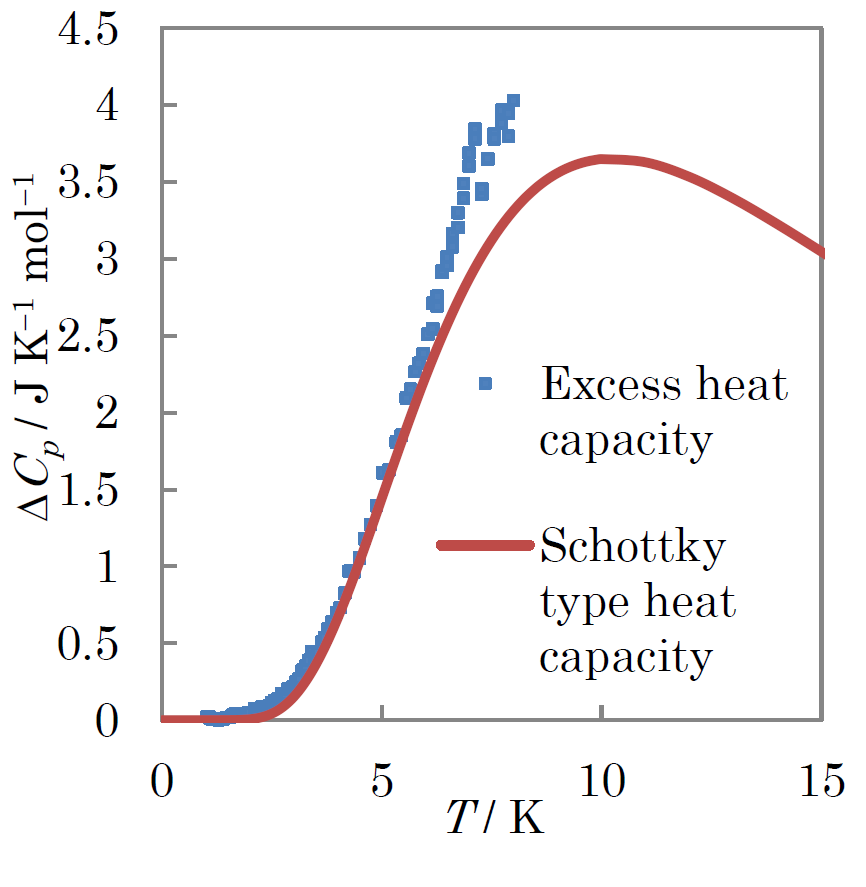
Fig.3.(Color online)Excess heat capacity of [ReIIICl2(PPr3)2 (Hbim)][ReIVCl2(PPr3)2(bim)] below 8 K in a Cp vs T plot.The solid curve stands for the Schottky type heat capacity.