|
|
|
|
|
|
|
|
 |
|
 |
|
1. Nano-Chemistry at Liquid-Liquid Interfaces |
|
|
|
|
So many reactions in biological and environmental systems are taking place at interfaces. We are developing new methods including a total internal reflection absorption /fluorescence/Raman method, a high-speed stirring method, a centrifugal liquid membrane method, a micro-sheath flow/laser excited spectrofluorometry, and a two-phase micro-flow TOF/MS for the measurements of interfacial reactions. Our interest covers a single molecule behavior at the interface, a fast interfacial reaction mechanism, an interfacial aggregation mechanism of metal complexes and an interfacial enzyme reaction mechanism.
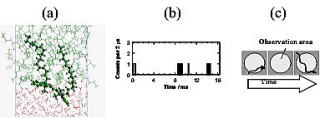
A single molecule probing is an ultimate method for measuring the properties of the nano-environment at the liquid-liquid interface. We have observed for the first time the single molecule diffusion at the liquid-liquid interface by means of the total internal reflection fluorescence microscopy. The diffusion coefficients and apparent interfacial viscosities were evaluated from the maximum duration of photon burst form the single molecule of DiI (Fig. 1). In the dodecane/water interface, the observed viscosity of 1.4 mPa.s was close to the value for dodecane and was higher than that of water, suggesting that the two long alkyl-groups of DiI are deeply immersed in the dodecane phase, as shown in Fig. 1. SDS did not have a significant effect on the diffusion of the single molecule. On the other hand, DMPC, a composite of biological cell membranes, had a remarkable effect. The viscosity in the DMPC interface was higher than the surfactant-free interface by two orders of magnitude.
|
|
|
 |
|
|
Fig.1 Single molecule lateral diffusion at the dodecane/water interface
; (a) A snapshot of the MD simulation of DiI, (b) Photon burst from DiI
with an average concentration of 0.02 molecules in the observation area,
and (c) A model for the photon burst observation in single molecule diffusion.
|
|
|
|
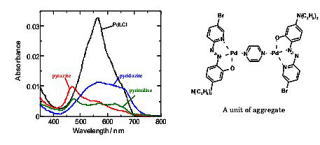
Interfacial adsorption of molecules or ions can produce a highly concentrated environment for the adsorbed species. We found out that the aggregates of metal complexes exhibited a unique function as molecular recognition reagents. The aggregated state is similar to a clustered state, in which the monomer molecules are closely packed in the two-dimensional interface. In such state, the selectivity in the structural recognition for isomers could be significantly enhanced (Fig. 2).
|
|
|
 |
|
|
Fig. 2 Molecular recognizing interfacial adsorption of the aggregate of diazine with Pd-5-Br-PADAP complex. The absorbance of the complex is remarkably reduced by the interfacial aggregation at toluene/water interface.
|
|
|
|
|
|
|
2. Development of Innovative Migration Analysis Utilizing External Fields |
|
|
|
|
For analyses of ions and molecules, there have been developed so many analytical
separation methods such as HPLC and CE. However, only a few methods are
developed so far for the separation of microparticles. We have invented
new migration methods for the analysis of microparticles in relation to
environmental and biological interests by utilizing various external fields.
A laser-photophoresis, a capillary dielectrophoresis, an electromagnetophoresis
and a magnetophoretic velocimetry have recently been developed as innovative
migration methods in our laboratory. We developed a capillary flow dielectrophoresis
with a quadrapole electrode inside for the analysis of DNA and biological
cell such as yeast cell. This achieved mutual separation of various sized
polystyrene particles by using only 2 cm quadrapole electrode capillary.
Lorentz force under 10 T magnetic field was used for the measurement of
adsorption force of a single particle in liquid to a silica capillary wall.
This innovative method using electromagnetophoretic buoyancy achieved the
sensitivity of a few pN, which was extremely more sensitive than that of
the ordinary AFM method. This method will be extended to invent a new electromagnetophoretic
chromatography. We have invented magnetophoretic velocimetry, which can
determine the magnetic susceptibility of a single micro-particle in liquid
and the amount of a component in a micro-particle, even in the system that
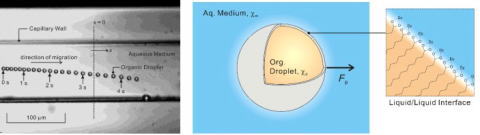
Dy(III) ion is adsorbed at the interface of a single organic droplet (Fig.3).
|
|
|
 |
|
|
Fig. 3 Magnetophoretic determination of Dy(III) adsorbed at the interface
of 2-fluorotoluene droplet in water .
|
|
|
|
|
|
|
Reference |
|
|
|
- "Lateral Diffusion Dynamics for Single Molecules of Fluorescent Cyanine Dye at Free and Surfactant-Modified Dodecane-Water Interface," F. Hashimoto, S. Tsukahara and H. Watarai, Langmuir, 19, 4197-4204 (2003).
- "Kinetic Study of Fast Complexation of Zinc(II) with 8-Quinolinol
and 5-Octyloxymethyl-8-quinolinol at 1-Butanol/Water Interface by Two-Phase
Sheath Flow/Laser-Induced Fluorescence Microscopy," T. Tokimoto, S.
Tsukahara and H. Watarai, Bull. Chem. Soc. Jpn., 76, 1569-1576 (2003).
- "In situ Measurement of Aggregate Formation Kinetics of Nickel(II)-Pyridylazoaminophenol
Complex at the Heptane-Water Interface by Centrifugal Liquid Membrane Spectrophotometry,"
Y. Yulizar, A. Ohashi and H. Watarai, Bull. Chem. Soc. Jpn., 76, 1379-1386
(2003).
- "Interfacial nanochemistry in liquid-liquid extraction systems",
H. Watarai, S. Tsukahara, H.
Nagatani and A. Ohashi, Bull. Chem. Soc. Jpn., (Accounts) 76, 1471-1492 (2003).
- "Magentophoretic Velocimetry of Microorganic Droplets Adsorbed by
Dysprosium(III) Laurate in Water," M. Suwa and H. Watarai, J. Chromatogr.
A, 1013, 3-8 (2003).
- "Total internal reflection resonance Raman microspectroscopy for the
liquid/liquid interface. Ion-association adsorptionof cationic Mn(II) porphine",
K. Fujiwara and H. Watarai, Langmuir, 19(7), 2658-2664(2003).
- "Microgravity laser-photophoresis of high density microparticles in
water", M. Tamagawa, H. Monjushiro and H. Watarai, Colloids Surf.
A:Pysicochem. Eng. Aspects, 220, 279-284 (2003).
|
|
|
|
|
|
|
|
|
|
|
|
|