|
|
|
|
|
|
|
|
 |
|
 |
|
1. Nano-Chemistry at Liquid-Liquid Interfaces |
|
|
|
|
Real chemical reactions in biological and environmental systems take place mainly at interfaces. We have been developing new methods to measure the reactions at liquid-liquid interfaces; including a high-speed stirring method, a centrifugal liquid membrane method, micro-sheath flow/laser spectrofluorometry, two-phase micro-flow TOF/MS and total internal fluorescence/Raman and second-harmonic generation (SHG) spectroscopy. We have studied a single moleculefs behavior, fast interfacial reaction kinetics, an interfacial aggregation, and an enzyme reaction at liquid-liquid interfaces.
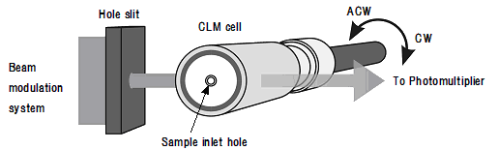
Now, we are focusing on the measurement of optical chirality at liquid-liquid interfaces. Optical chirality has been investigated not only in chemistry, but also in physics and biology. However, the liquid-liquid interface has never become a focus of the research on optical chirality; because of the difficulty of measurement, although better understanding would be very important for the development of chiral recognition and chiral separation of drugs and biological molecules at an interface. We have developed new methods for the measurement of optical chirality at liquid/liquid interfaces including centrifugal liquid membrane (CLM)-circular dichroism (CD) spectrometry (Fig. 1) and second harmonic generation (SHG)-circular dichroism (CD) spectrometry. Furthermore, these methods have been combined with an optical microscope to achieve the chiral measurement of a micro-area or a single microparticle. By these new methods, the characteristic features of the optical chirality of the aggregates of porphyrin and phthalocyanine and the bilirubin-bovine serum albumin (BSA) complex formed at a liquid-liquid interface have been investigated. The aggregate of chiral thioether-substituted phthalocyanine bound by Pd(II) showed optical chirality that depended on the chirality of the peripheral thioether, although the phthalocyanine showed no chirality in the visible spectrum. The chiral J-aggregation of an achiral hydrophobic porphyrin was investigated with CLM-CD and SHG-CD. Tetraphenylporphyrin formed an aggregate of di-protonated porphyrin at a toluene/acid interface. The chirality of the aggregate was governed by a chiral counter-anion, such as camphor-10-sulfonate, or the chiral hydrophobic alcohol, such as 2-nonanol. Bilirubin bound to BSA at the liquid-liquid interface increased the proportion of its M(-) form (Fig. 2).
|
|
|
 |
|
|
Fig.1 Schematic of the centrifugal liquid membrane (CLM) method combined
with circular dichroism polarimetry
|
|
|
|
|
|
|
|
Fig. 2 M conformer increased by the formation of a 1:1 bilirubin-BSA complex at the heptane/water interface, measured by CLM-CD.
|
|
|
|
|
|
|
2. Development of Innovative Microparticle Analysis Using External Fields |
|
|
|
|
In modern analytical chemistry, the analysis of microparticles and interfaces is a most attractive subject in view of the need to analyze biological and environmental microparticles. We have invented new migration-based analytical methods for microparticles that use various external physical fields. Techniques such as laser-photophoresis, capillary dielectrophoresis, electromagnetophoresis, and magnetophoretic velocimetry have recently been developed in our laboratory. We are now developing the following new methods using magnetic fields in the analysis of microparticles :
|
1. |
Magnetophoretic velocimetry of microparticles in liquids
|
|
|
The migration velocity of microparticles in liquid under a magnetic field
gradient has been analyzed. For example, the velocity of a 2-fluorotoluene
droplet including a hydrophobic carboxylic acid in a diluted Dy(III) solution
is related to the magnetic susceptibility of the interface due to the formation
of a Dy(III) complex at the interface. Magnetophoresis is a promising method
for the detection of a change of the spin state in a single microparticle,
which may be induced by photochemical reaction or redox reaction.
|
|
|
2. |
Magnetic force mass analysis under ambient conditions
|
|
|
Conventional mass spectrometry (MS) requires sample molecules to be ionized, which is sometimes a drawback, because it induces undesirable fragmentation of the sample molecules. Using magnetic force to drive the migration of a microparticle under atmospheric pressure, we have invented a new class of MS, which can give the magnetic susceptibility and mass of a single microparticle simultaneously.
|
|
|
3. |
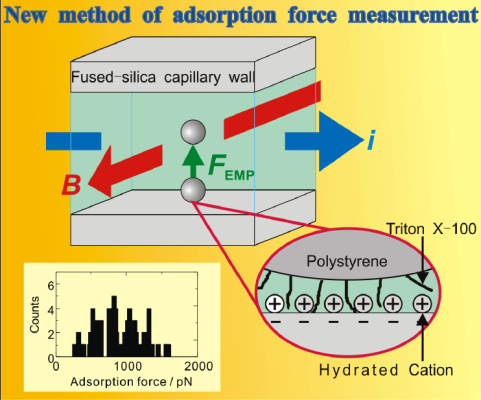
Electromagnetophoresis of microparticles (Fig. 3)
|
|
|
Electromagnetic force generating electromagnetic buoyancy in an electrolyte solution was used to mobilize a microparticle in the solution. The force required to desorb a microparticle adsorbed on a capillary wall can be measured with a controlled electromagnetic force. This principle was used to measure the force of a single chemical bond between a microparticle (e.g. a yeast) and Con A in a wall. Furthermore, this principle is the basis of a new analytical technique: adsorption-desorption chromatography of microparticles.
|
|
|
|
|
 |
|
|
Fig. 3 Electromagnetophoretic determination of the chemical force binding a single particle.
|
|
|
|
|
|
|
Reference |
|
|
|
- gOptical chirality of protonated tetraphenylporphyrin J-aggregate formed
at the liquid-liquid interface in a centrifugal liquid membrane cellh,
S. Wada, K. Fujiwara, H. Monjushiro and H. Watarai, J. Phys.:Condens. Matter,
19, 375105(12pp) (2007).
- gResonance Raman spectroscopic study on chiral aggregation of bilirubin-bovine
serum albumin complex formed at liquid/liquid interfaceh, J-H. Yin and
H. Watarai, Anal. Sci., 23, 841-846 (2007).
- gEffects of a magnetic force on surface-enhanced Raman spectra of a cysteamine
linking magnetic particle and a silver colloid plateh, T. Goto, S. Yamamoto
and H. Watarai, Anal. Sci., 23, 891-893 (2007). (Hot Article)
- gElectromagnetophoretic force measurement of a single binding interaction
between lectin and yeast cells surfacesh, Y. Iiguni and H. Watarai, Anal.Sci.,
23, 121-126 (2007).
- (5) gIn situ measurement of individual W/O microemulsions of aerosol OT in dodecane/water extraction system by total internal reflection laser light scattering microscopyh, S. Tsukahara, H. Kitaguchi and H. Watarai, Chem. Lett., 36, 148-149 (2007).
|
|
|
|
|
|
|
|
|
|
|
|
|