Coordination Chemistry Laboratory, Department of Chemistry, Graduate School of Science, The University of Osaka
Exploitation of Coordination Molecular Technology That Leads to the Creation of New Conceptual Ionic Solids with New Functionalities
The digold(I) complex having 1,2-bis(diphenylphosphino)ethane, [Au2(DHpen)2(dppe)], serves as a chiral bis(tridentate-N,O,S) metalloligand toward CoIII to form a cationic S-bridged AuI4CoIII2 hexanuclear complex, [Au4Co2(dppe)2(D-pen)4]2+. Remarkably, this hexanuclear complex was found to crystallize with ClO4- to form metallosupramolecular ionic crystals, in which 6 AuI4CoIII2 complex-cations are self-assembled to form a big octahedral supramolecule, with the concomitant aggregation of 10 ClO4- anions into an amazing adamantine-like anionic cluster.
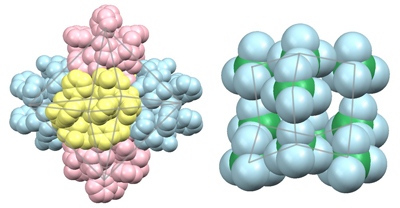
Hexamer of AuI4CoIII2 complex-cations (left) and decamer of 10 ClO4- anions (right)
Please check the web site of our research project [link]
