Coordination Chemistry Laboratory, Department of Chemistry, Graduate School of Science, The University of Osaka
Creation of Metalloaggregates Based on Metalloligand
The design and creation of homometallic and heterometallic molecular aggregates that possess unique structures and properties have attracted increasing attention. While the most common approach to creating metalloaggregates is the use of functional organic ligands that can bridge two or more metal centers, our efforts have concentrated on the use of thiolato metal complexes as an S-donating metalloligand.
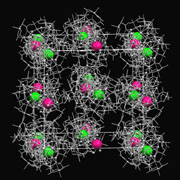 We have shown that [Au(D-Hpen-S)2]- (D-H2pen = D-penicillamine) reacts with AgI to produce
a photoluminescent AuI2AgI2 complex, which further reacts with CuII in the presence of Cl- to produce a metallo-supramolecular compound containing all three coinage metals. Remarkably, this product was found to be composed of monocationic 20-nuclear AuI6AgI8CuII6
and monoanionic 21-nuclear AuI6AgI9CuII6
supramolecular cages, which are coupled to each other to form an unprecedented
1:1 supramolecular salt with a 'rock-salt'-like lattice structure.
We have shown that [Au(D-Hpen-S)2]- (D-H2pen = D-penicillamine) reacts with AgI to produce
a photoluminescent AuI2AgI2 complex, which further reacts with CuII in the presence of Cl- to produce a metallo-supramolecular compound containing all three coinage metals. Remarkably, this product was found to be composed of monocationic 20-nuclear AuI6AgI8CuII6
and monoanionic 21-nuclear AuI6AgI9CuII6
supramolecular cages, which are coupled to each other to form an unprecedented
1:1 supramolecular salt with a 'rock-salt'-like lattice structure.
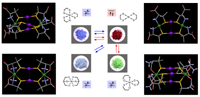 In addition,
we have shown that red AuI2NiII2,
purple AuI3NiII2, light blue AuI2NiII2,
and green AuI3NiII2 complexes are
created only from [Au(D-pen-S)2]3? in
combination with NiII. It was found that the red complex is triply
reversible with the purple, light blue, and green complexes in response to NiII:[Au(D-pen-S)2]3- stoichiometry and solution pH,
accompanied not only by the readily detectable color change, but also by
drastic switches in magnetism and chirality.
In addition,
we have shown that red AuI2NiII2,
purple AuI3NiII2, light blue AuI2NiII2,
and green AuI3NiII2 complexes are
created only from [Au(D-pen-S)2]3? in
combination with NiII. It was found that the red complex is triply
reversible with the purple, light blue, and green complexes in response to NiII:[Au(D-pen-S)2]3- stoichiometry and solution pH,
accompanied not only by the readily detectable color change, but also by
drastic switches in magnetism and chirality.
For detailed information, please see our account:
A. Igashira-Kamiyama & T. Konno, Dalton Trans., 2011, 40, 7249-7263 (Perspective)
[link]
