Electronic Heat Capacity
of a Single-Component Molecular Conductor
We have performed a thermodynamic investigation of a single component molecular
conductor [Au(tmdt)2] in order to confirm the
realization of a metal ground state. This material is designed and synthesized
by the strategy to decrease HOMO-LUMO gap and increase the overlaps of
neighboring molecules in the crystalline structure by Profs. A. Kobayashi and
H. Kobayashi. The heat capacity measurements were performed by the relaxation
calorimetry technique using compressed pellet samples at low temperatures.
Although some traces of paramagnetic impurities were observed by studying the
magnetic fields dependence, the existence of a T-linear
term which is not affected by the external fields was confirmed. The electronic
heat capacity coefficient γ is estimated to be
about 10 mJ K−2 mol−1, which is consistent with the result of the
paramagnetic susceptibility measurement. The observation supports the
realization of metallic ground state in this compound.
(by Y. Inoue & Y. Nakazawa)
|
Fig. 1.
Molecular structure of [M(tmdt)2],
where tmdt is trimethylenetetrathiafulvalenedithiolate.
|
|
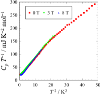
Fig. 2.
Temperature dependence of the heat capacity of
[Au(tmdt)2] obtained by a compressed pellet sample.
The broad hump observed around 4 K is considered as an extra contribution of
phonons owing to the the libration of stacked molecules.
|
|
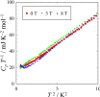
Fig. 3.
Low-temperature heat capacity of
[Au(tmdt)2] shown as a
Cp T−1 vs
T2 plot.
The existence of electronic heat capacity coefficient of about
10 mJ K−2 mol−1 is observable.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.

