Research 1
Excess Heat Capacity by Partial Deuteration of Methyl Groups in Toluene
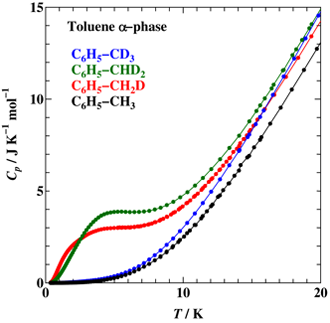
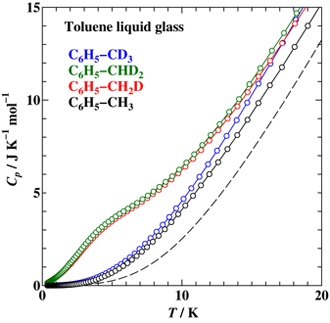
Heat capacity measurements were made for toluene (C6H5–CH3) and its methyl-deuterated analogs, C6H5–CH2D, C6H5–CHD2, and C6H5–CD3, between 0.35 K and 200 K by adiabatic calorimetry and relaxation calorimetry. An excess heat capacity with a broad maximum was found below 20 K for α phase (stable crystal) of C6H5–CH2D and C6H5–CHD2. Unlike in the case of 2,6-dichlorotoluene, the contribution is not explained by any simple energy schemes involving three levels. The entropy is significantly less than Rln3 (= 9.13 J K-1 mol-1). This indicates that the orientational disorder of –CH2D (or –CHD2) is frozen in at lower temperatures. The glass of liquid C6H5–CH2D and C6H5–CHD2 also showed an excess heat capacity at low temperatures. But, the shape is much broader than that of the α phase.
(by H. Suzuki & A. Inaba)
|
Fig. 1.
Low temperature heat capacity of toluenes in the α phase (stable crystal). The partially deuterated compounds (–CH2D and –CHD2) showed a broad anomaly.
|
|
Fig. 2.
Low temperature heat capacity of toluenes in the glass. The –CH2D and –CHD2 compounds showed an anomaly which is much broader than that of α phase. The broken curve stands for the heat capacity of fully protonated toluene (–CH3) in the α phase.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.

