Research 2
Excess Heat Capacity by Partial Deuteration of Methyl Groups in 2,6-Dibromotoluene
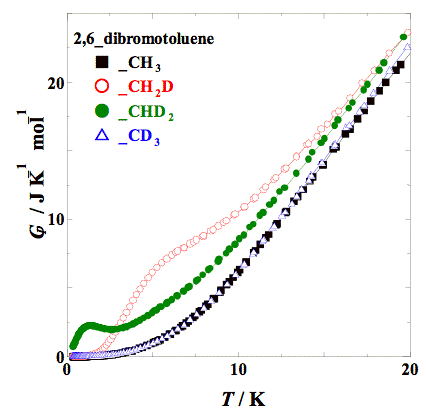
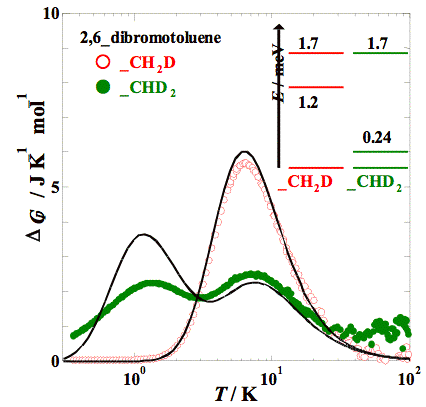
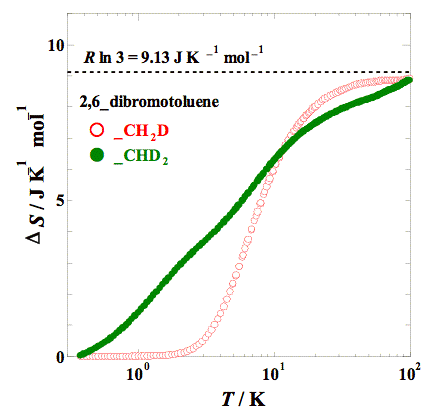
Heat capacity measurements were made for 2,6–dibromotoluene and its methyl–deuterated analogs (–CH2D, –CHD2, and –CD3) by adiabatic calorimetry (5 – 300 K) and relaxation calorimetry (0.35 – 20 K). The partially deuterated compounds (–CH2D and –CHD2) showed an anomaly in heat capacity at low temperatures, which is similar to that obtained for 2,6–dichlorotoluenes. The excess entropy is close enough to the value expected, ΔS = R ln 3 (= 9.13 J K−1 mol−1) for both compounds. The excess heat capacity can also be fitted with a Schottky–type function with three levels.
(by K. Yoshida, H Suzuki & A. Inaba)
|
Fig. 1. Low temperature heat capacities of 2,6–dibromotoluenes. The partially deuterated compounds (–CH2D and –CHD2) showed a broad anomaly.
|
|
Fig. 2. Excess heat capacities of partially deuterated 2,6–dibromotoluenes, –CH2D and –CHD2. Solid curves stand for the Schottky-type heat capacities obtained by the energy scheme shown.
|
|
Fig. 3. Excess entropies of partially deuterated 2,6–dibromotoluenes, –CH2D and –CHD2. Dashed line indicates the value of ΔS = R ln 3 (= 9.13 J K−1 mol−1).
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.


