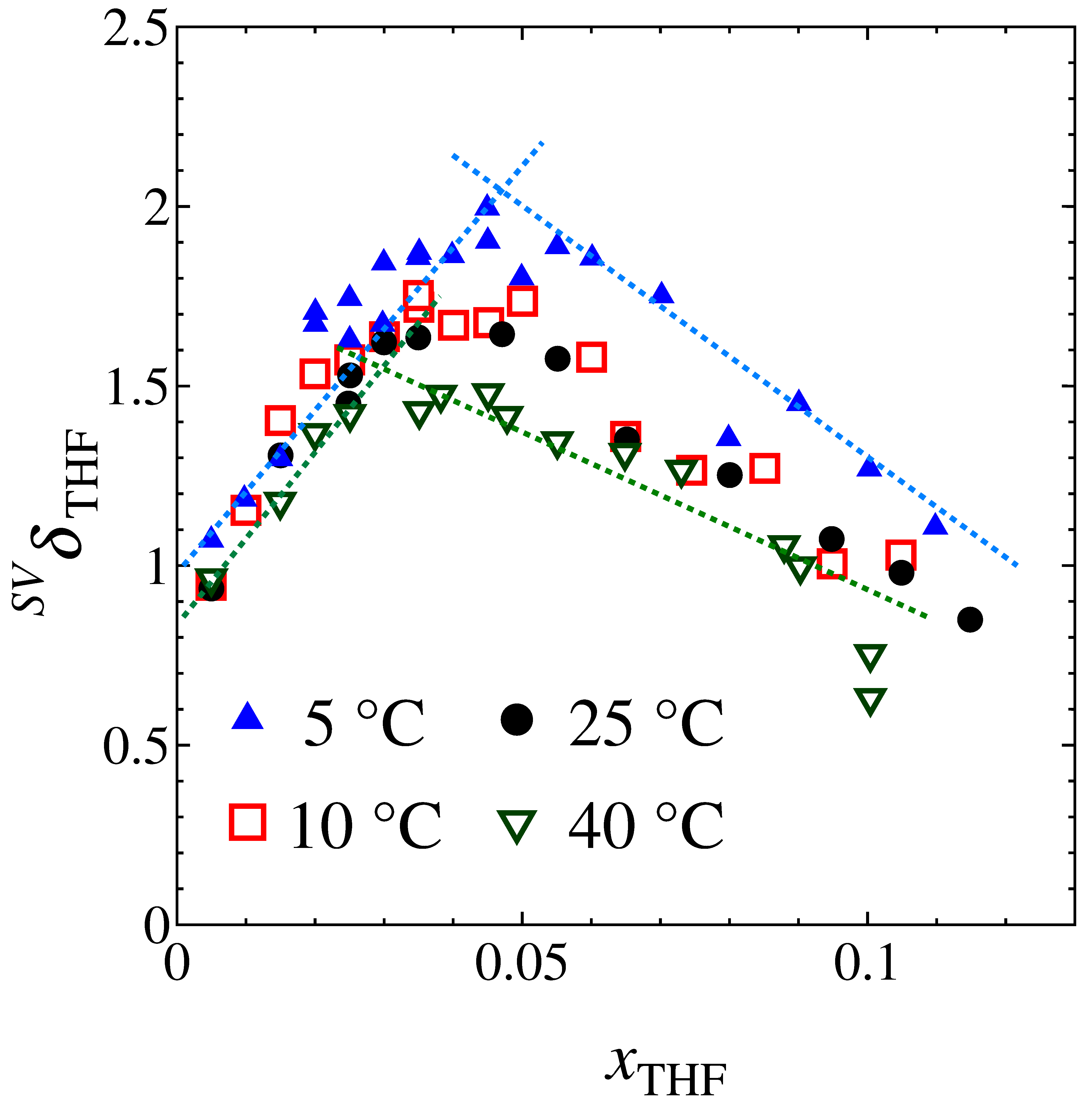
Fig. 1. Color online. Mole fraction dependence of SVδTHF of aqueous THF at different temperatures from 5 °C to 40 °C.
From our previous studies, we found that some third derivatives of Gibbs energy for a variety of aqueous solutions show anomalous behaviors. For aqueous solution of hydrophobic solute, the pattern exhibits a peak-type anomaly, and for a hydrophilic solute a bend-type. We found that the points at the peak or bend are the boundary of two adjacent Mixing Schemes. Below its mole fraction, the percolation of hydrogen bond is still retained, but at higher mole fraction the percolation is broken. The solution then consists of water-rich and solute-rich clusters. In order to gain an in-depth insight at the Mixing Schemes, we chose tetrahydrofuran (THF) as a solute. The THF aqueous solution forms a clathrate hydrate with xTHF = 0.056 below 4.6 °C, and its property and structure are well studied. When the clathrate hydrate melts above 4.6 °C, THF could form a hydration shell with similar structure of the clathrate hydrate. As the first step, we measured SVδTHF, the partial molar density entropy-volume cross fluctuation of solute, directly for THF aqueous solution.
The results of SVδTHF measurement plotted in Fig.1 shows a peak-type anomaly. This is typical of a hydrophobic solute. The relationship between the mole fraction and the temperature at the anomalies is shown in Fig. 2 (•) together with those for other solutes. The data points of the THF aqueous solution also forms "the Koga line". Furthermore, the line seems to point to xTHF = 0.056 at low temperature. This suggests that the solution below Koga line is preparing to form the clathrate on cooling down to 4.6 °C. Hence the solution must have hydration shell around a single THF in the dilution region. This supports our interpretation that the Koga line is the boundary of two Mixing Schemes. The extrapolated value of temperature to zero mole fraction of the line seems to be 70 °C. As evident in Fig. 2, all other cases also seem to point to about 70 °C, regardless of the hydrophobicity/hydrohpilicity of solute. This suggests that the bond percolation of hydrogen bond is broken at about 70 °C even for pure water.

Fig. 1. Color online. Mole fraction dependence of SVδTHF of aqueous THF at different temperatures from 5 °C to 40 °C.
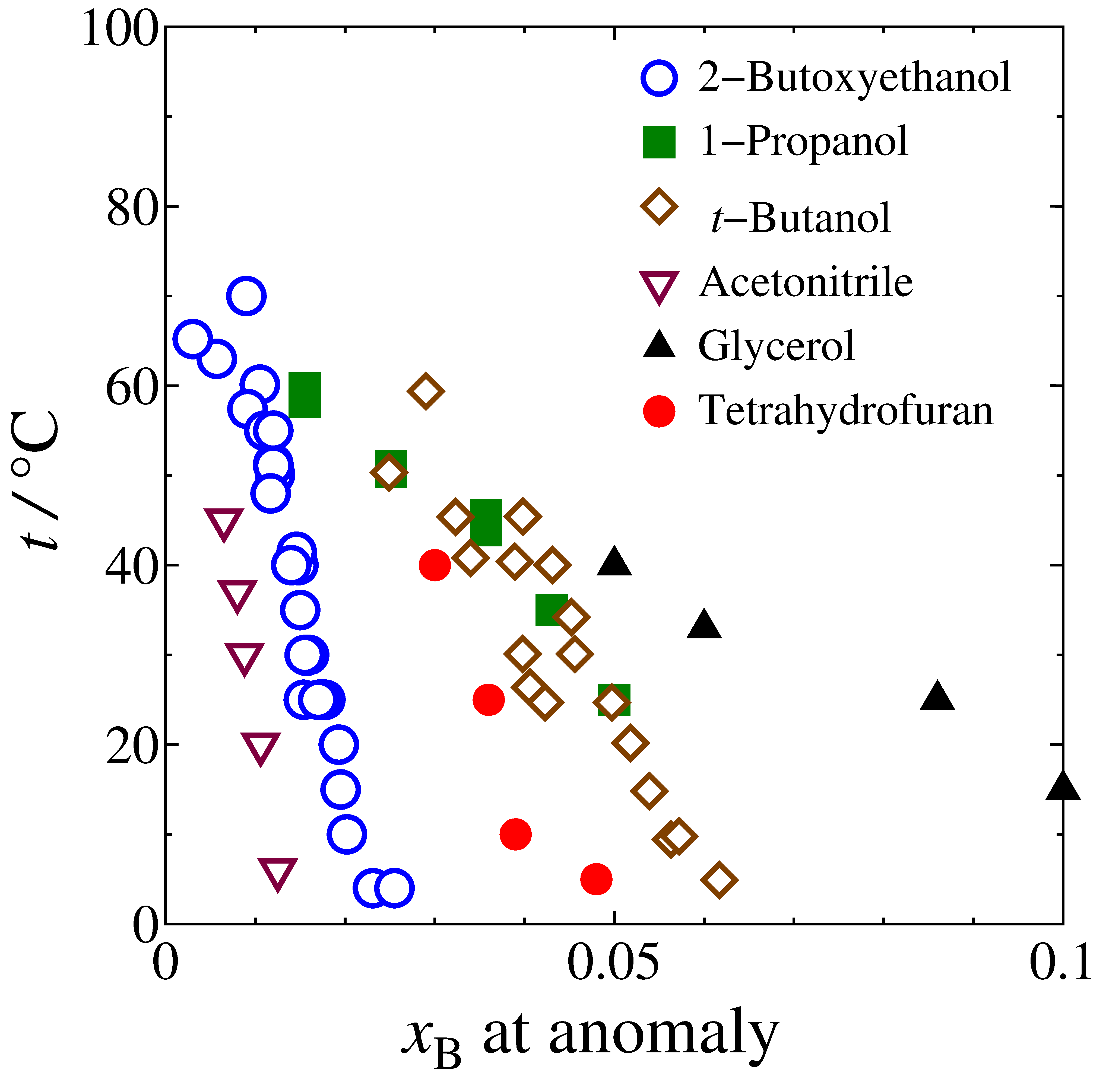
Fig. 2. Color online. Loci of the anomaly points obtained for various solutes.