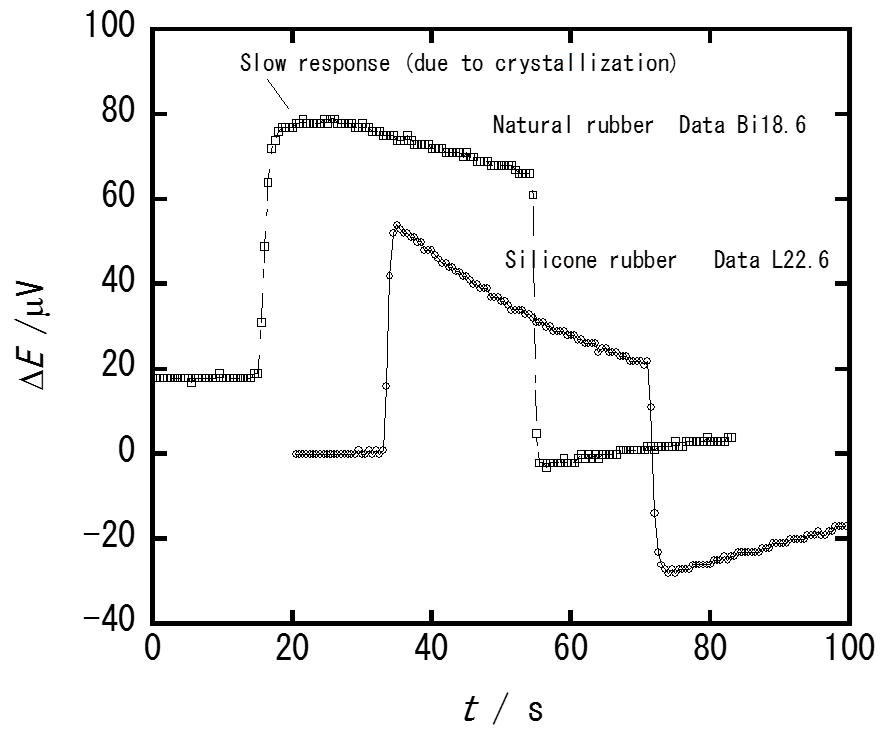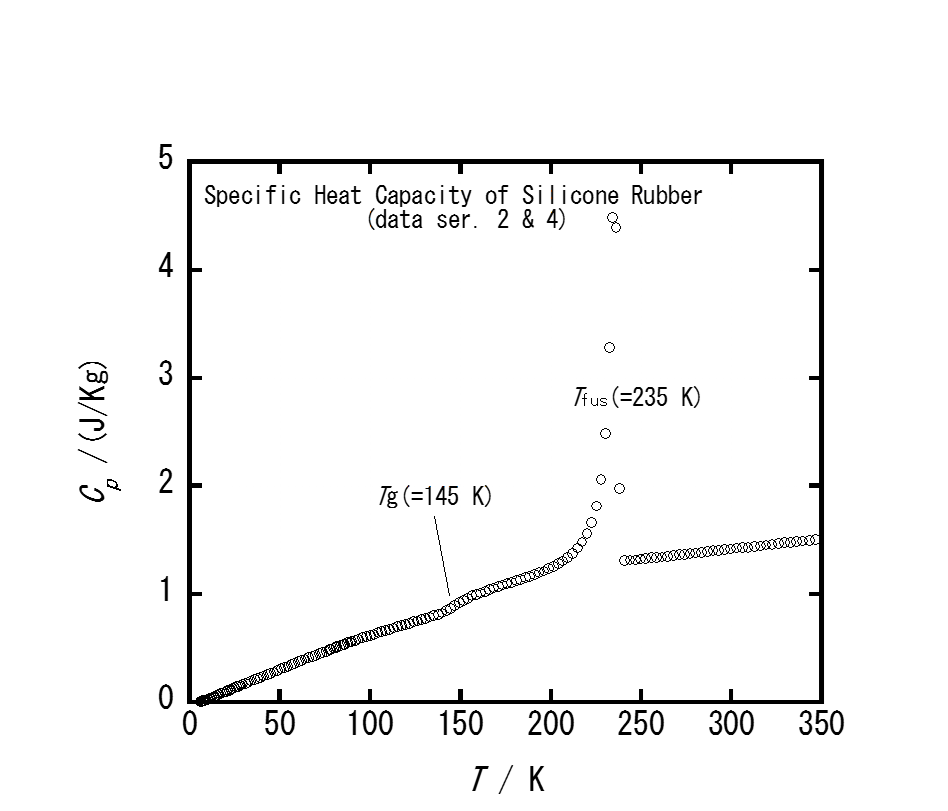Research 4
The Mechanocaloric Effect and
Glass Transition in Silicone Rubber
Temperature change of a piece of silicone rubber was measured with a thin wire thermocouple sandwiched between the sample rubber, while it was stretched or allowed to shrink. The temperature increased on elongation and decreased on contraction by ca. 1 K for a 400 % change of the length. The temperature change known as the mechano-caloric effect arises from thermodynamic coupling between the temperature and mechanical deformation of the rubber through vibrational and conformational entropy of the polymer chain molecules. Heat capacity measurement has shown that the rubber undergoes a glass transition at 145K and fusion at 235K. By the use of the heat capacity and mechano-caloric data, the entropy of the rubber was evaluated as a function of elongation, and related to the average number of polymer segments between adjacent cross-linking points.
(by T. Matsuo & A. Inaba)
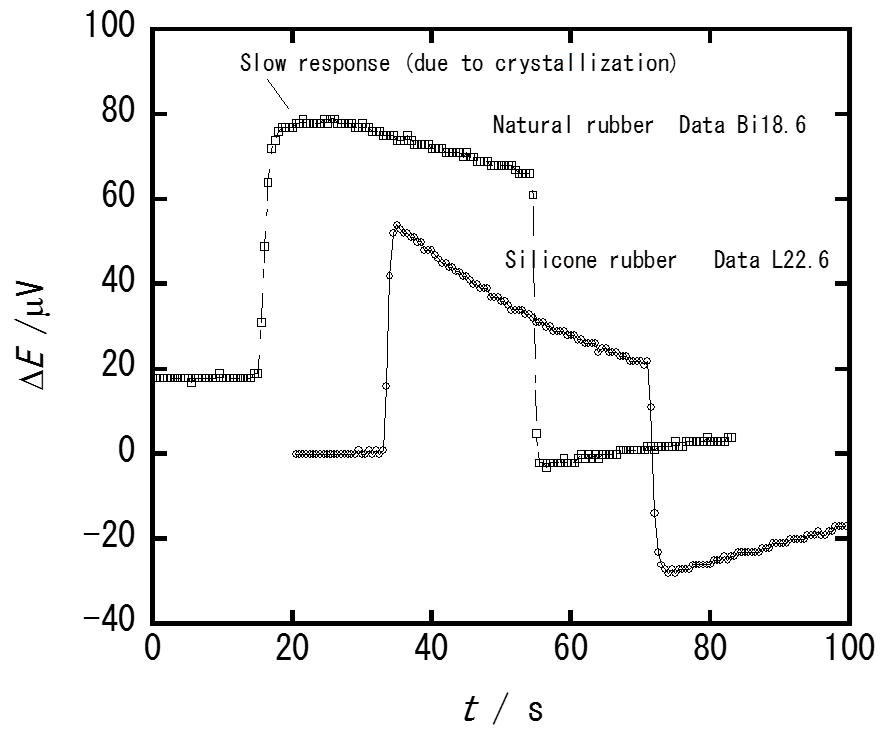
Fig. 1@Thermocouple output signals arising from the mechano-caloric response of silicone rubber and natural (isoprene) rubber to elongation and contraction at 301 K. The elongation ratio was ca. 4 and temperature changes about 1 K with the thermocouple sensitivity of 61.6 μV/K.
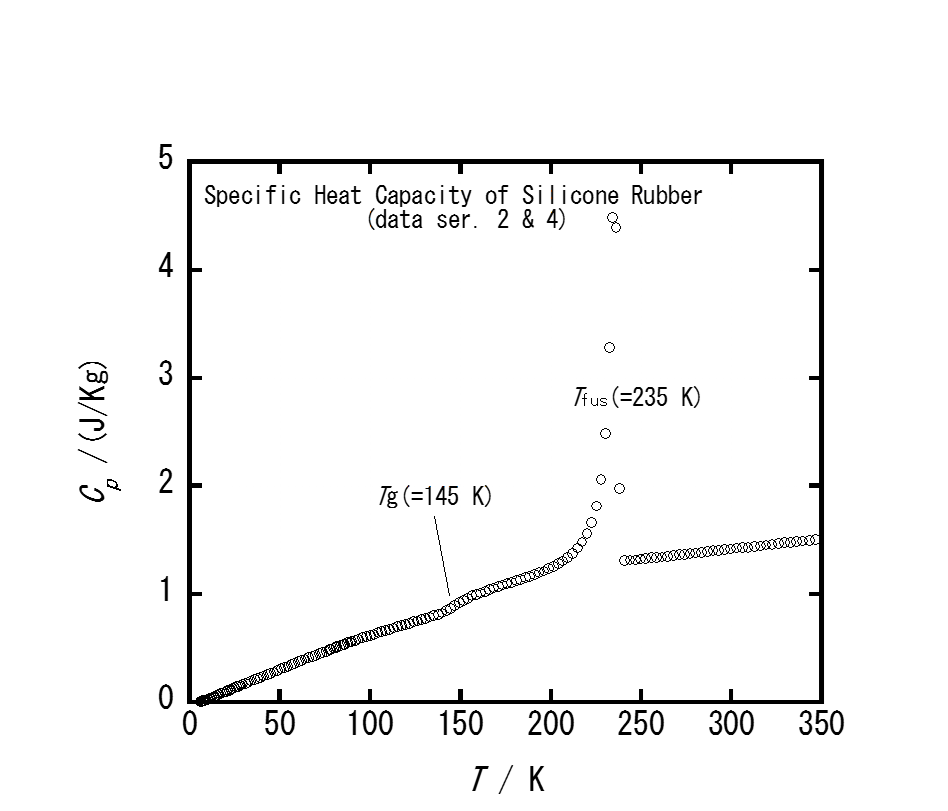
Fig. 2.Specific heat capacity of silicone rubber for temperatures between 5 and 350 K showing the glass transition and fusion.
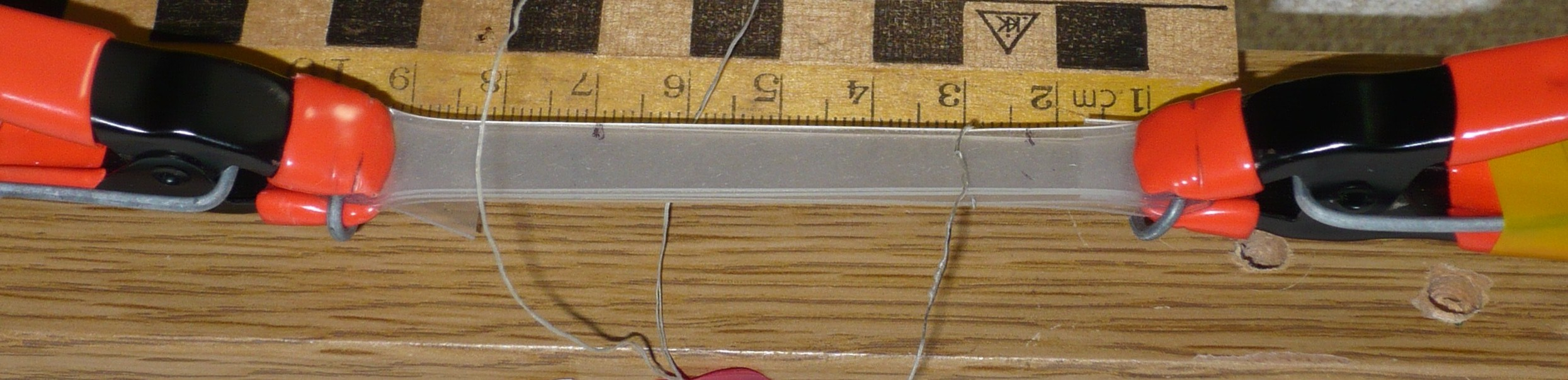
Photo 1. The silicone rubber sample under the mechano-caloric measurement. The length scale is shown by the centimeter ruler.
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.