Heat Capacity and Magnetic Phase Transition of the Molecule-Based Magnet
NNDPP• + · FeIII Br4−
Heat capacities of two crystal polymorphs (crystals A and B) of
2-(nitronyl nitroxide)-9,10-dipheny-9,10-dihydrolphenazine radical cation
NNDPP• +
(S = 1) and
FeIII Br4−
(S = 5/2) salt
NNDPP• + · FeIII Br4−
were measured by relaxation method under magnetic fields. Crystals A and B
exhibited heat capacity peaks due to magnetic phase transition at
3.38 K and 6.74 K, respectively. From the magnetic field dependences of
these magnetic phase transition, crystal A is antiferromagnetic and crystal B
is ferromagnetic. The magnetic entropies of crystals A and B were evaluated
to be 24.0
J K−1 mol−1
and 23.8
J K−1 mol−1, respectively, which are very close to
Rln(3×6) (= 24.0
J K−1 mol−1) for
S = 1 and S = 5/2 spin systems.
The zero-field magnetic heat capacities of crystals A and B above the
magnetic phase transition temperatures are expressed well by the theoretical
heat capacity curve for the high-temperature expansion of an
S = 3/2 one-dimensional antiferromagnetic
Heisenberg model with the intrachain magnetic interaction
J/kB
= −2.0 K and that of an
S = 5/2 one-dimensional antiferromagnetic
Heisenberg model with the intrachain magnetic interaction
J/kB
= −1.0 K, respectively.
From the mean-field approximation by use of the derived intrachain
magnetic interactions and the magnetic phase transition temperatures,
the interchain magnetic interactions for both crystals were estimated to be
|zJ′/kB|
≈ 0.28 K.
(by X.-Z. Lan & Y. Miyazaki)
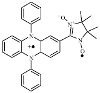
|
Fig. 1.
Molecular structure of NNDPP• +.
|

|
Fig. 2.
Magnetic heat capacities of
NNDPP• + · Fe Br4−
(crystals A (top) and B (bottom)) under magnetic fields.
For the sake of clarity, the magnetic heat capacities except for the
zero-field magnetic heat capacities are shifted upwards. Solid curves
indicate the theoretical heat capacity for high-temperature expansion of
S = 3/2
one-dimensional antiferromagnetic Heisenberg model with
J/kB
= −2.0 K for crystal A and that of
S = 5/2
one-dimensional antiferromagnetic Heisenberg model with
J/kB
= −1.0 K for crystal B, respectively.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.

