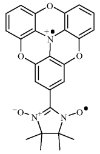
Fig. 1. Molecular structure of NNTOT• +.

Fig. 2. Magnetic heat capacities of NNTOT• + · Fe Cl4− (top) and NNTOT• + · Ga Cl4− (bottom) under magnetic fields. For the sake of clarity, the magnetic heat capacities except for the zero-field magnetic heat capacities are shifted upwards. Solid curves indicate the theoretical heat capacity for high-temperature expansion of S = 5/2 one-dimensional antiferromagnetic Heisenberg model with J/kB = −0.18 K (green), that of S = 1 one-dimensional antiferromagnetic Heisenberg model with J/kB = −1.9 K (red), and their summation (blue) for NNTOT• + · Fe Cl4− (top), and that of S = 1 one-dimensional antiferromagnetic Heisenberg model with J/kB = −1.9 K (red) for NNTOT• + · Ga Cl4− (bottom), respectively.