Thermodynamic Property of 1D Strongly Correlated Charge-Ordered Complex,
(DI-DCNQI)2Ag under Pressures
We have measured the heat capacities of organic charge transfer salts of
(DI-DCNQI)2Ag by the ac-modulation technique
under pressures and magnetic fields. In this salt, the DCNQI molecules
stack along the c-axis and form one-dimensional
conducting columns in this direction. These 1D columns are bridged by
monovalent metal cation M = Ag through a CN-M coordination bond.
(DI-DCNQI)2Ag forms a charge-ordered state
around 220 K and shows antiferromagnetic order around 6 K. We have observed
that the heat capacities are reduced gradually under pressures of
5 kbar, 10 kbar, and 16 kbar, which suggests that the charge-ordered state
is suppressed by applying pressures. External pressure is useful parameter
for studying these organic charge transfer salts.
(by N. Tokoro & Y. Nakazawa)
|
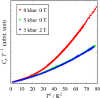
Fig. 1.
Cp T−1 vs.
T2
plot of
(DI-DCNQI)2Ag at ambient pressure
and under pressure of 5 kbar. We can see reduction of the heat capacity
with increasing pressures. This tendency is consistent with the picture
that the external pressure suppresses the charge-ordered state.
|
|
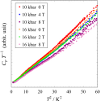
Fig. 2.
Cp T−1 vs.
T2
plot of
(DI-DCNQI)2Ag under pressure of
10 kbar and 16 kbar. The field dependence of the heat capacities
with applying pressures is observed.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.

