Coordination Chemistry Laboratory, Department of Chemistry, Graduate School of Science, The University of Osaka
Chiral Recognition and Aggregation of Metal Complexes
Considerable attention has been paid to the rational synthesis of chiral metal compounds in the field of coordination stereochemistry. This has been stimulated by the growing interest in the design and creation of well-organized metallo-supramolecular species, the overall structures of which can be controlled by the chirality of their building blocks. Our research interest has been directed toward this subject, focusing on the metal-assisted aggregation of chiral complex-units containing aminothiolate-type ligands.
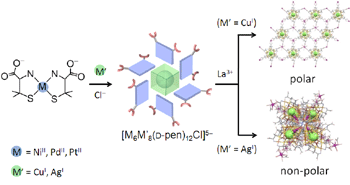 Recently we have reported the rational synthesis of a series of S-bridged 14-nuclear clusters with twelve free carboxylate groups, [MII6M'I8(D-pen)12Cl]5-, by employing [MII(D-pen-N,S)2]2- (MII = NiII, PdII, PtII) as the starting complex, in combination with thiophilic M' ions (M' = CuI, AgII). By reacting with La3+, the MII6CuI8 clusters formed polar 2D-sheet strcutures, while the MII6AgI8 clusters formed non-polar 1D-helix structures.
Recently we have reported the rational synthesis of a series of S-bridged 14-nuclear clusters with twelve free carboxylate groups, [MII6M'I8(D-pen)12Cl]5-, by employing [MII(D-pen-N,S)2]2- (MII = NiII, PdII, PtII) as the starting complex, in combination with thiophilic M' ions (M' = CuI, AgII). By reacting with La3+, the MII6CuI8 clusters formed polar 2D-sheet strcutures, while the MII6AgI8 clusters formed non-polar 1D-helix structures.
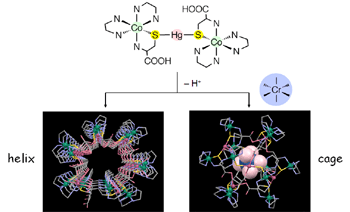 We synthesized an S-bridged CoIIIHgIICoIII complex having free carboxy groups by the reaction of chiral CoIII Complex ([Co(L-cys-N,S)(en)2]+) with HgII ion under strong acidic conditions. By the deprotonation of carboxyl groups, the trinuclear complex self assembled into three kinds of supramolecular assembledges (dimer, cage-type hexamer, and helix). In addition, the formation of these assemblages was able to be controlled by solution pH, presense/absence of metal ion, and/or chirality of the CoIII complex.
We synthesized an S-bridged CoIIIHgIICoIII complex having free carboxy groups by the reaction of chiral CoIII Complex ([Co(L-cys-N,S)(en)2]+) with HgII ion under strong acidic conditions. By the deprotonation of carboxyl groups, the trinuclear complex self assembled into three kinds of supramolecular assembledges (dimer, cage-type hexamer, and helix). In addition, the formation of these assemblages was able to be controlled by solution pH, presense/absence of metal ion, and/or chirality of the CoIII complex.
