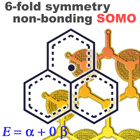


Phenalenyl is a thermodynamically stabilized hydrocarbon radical that possesses a non-bonding singly occupied molecular orbital (SOMO). Due to its high redox activity and an efficient orbital overlap between molecules, phenalenyl is considered to be a potential candidate for single component organic metals. In this context, we concern the following research themes; 1) intermediately dissociated electron pair (singlet biradical), 2) understanding the nature of multicenter bonding (pancake bonding), 3) molecular-based hydrocarbon metals, on the basis of the syntheses and investigation of novel phenalenyl-based radicals.

The aim of this research is to understand "edge-state" and "Dirac point", which are prominent features of graphene, at the molecular level by preparing and investigating small aromatic compounds, and also to explore electronic properties associated with those characteristic electronic structures of graphene. The synthetic target compounds for "edge-state" are PERIACENEs and for "Dirac point" three-fold symmetric DELTAPHANEs.

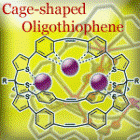
Cage-shaped oligothiophenes are expected to behave as host molecules that can encapsulate small molecules or metal ions. We prepare novel oligothiophenes based on tris(2-thienyl)methane and investigate their functional properties.

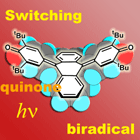
Non-planar pi-extended quinones, whose unique structure comes from the interplay between pi-conjugation and steric strain, are good candidates for external stimuli responsive materials. We are developing novel quinone molecules that possess many stable conformations.

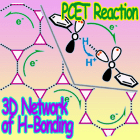
Proton-coupled electron transfer (PCET) reactions are the key steps in a variety of chemical and biological electron transfer processes. In such reactions, a proton and an electron are transferred together. We tried to apply these reactions to the electron transfer mechanism for conductive materials.

To create a novel proton-electron mediator, we design and synthesize new molecules which can stably trap a proton and an electron (or a hydrogen atom), and reversibly release them. We plan to apply this mediator for various PCET reactions. In addition, we try to realize functional materials of using PCET reactions, such as a photo-induced PCET in organic crystals.

In general, π-electrons are two-dimensionally delocalized on the aromatic system via covalentbonds. On the other hand, by arranging the aromatic systems to three-dimensions and congesting them as much as possible, the π-electrons can be delocalized in the space between aromatic rings and show unique properties which is not shown in two-dimensional aromatic hydrocarbons. In this work, by employing acene unit for the construction of π-congested aromatic systems, we have focused on the properties of space-delocalized π-electrons as well as the extremely long carbon-carbon single bonds which is probably formed between acene units by light irradiation.

The concept of “aromaticity” and “antiaromaticity” is crucial for the prediction and evaluation of electronic properties and stability of aromatic hydrocabons. Therefore, to design and create new functional materials based on the aromatic hydrocarbons, the concept is indispensable. In this work, we have focused on 1) stabilization and isolation of unstable aromatic 10π annulene as well as construction of unique hydrocarbon frameworks using the high reactivity of 10π annulene, 2) synthesis of new antiaromatic cyclooctatetraenes by employing new strategies toward a controllable antiaromaticity using external stimuli.
