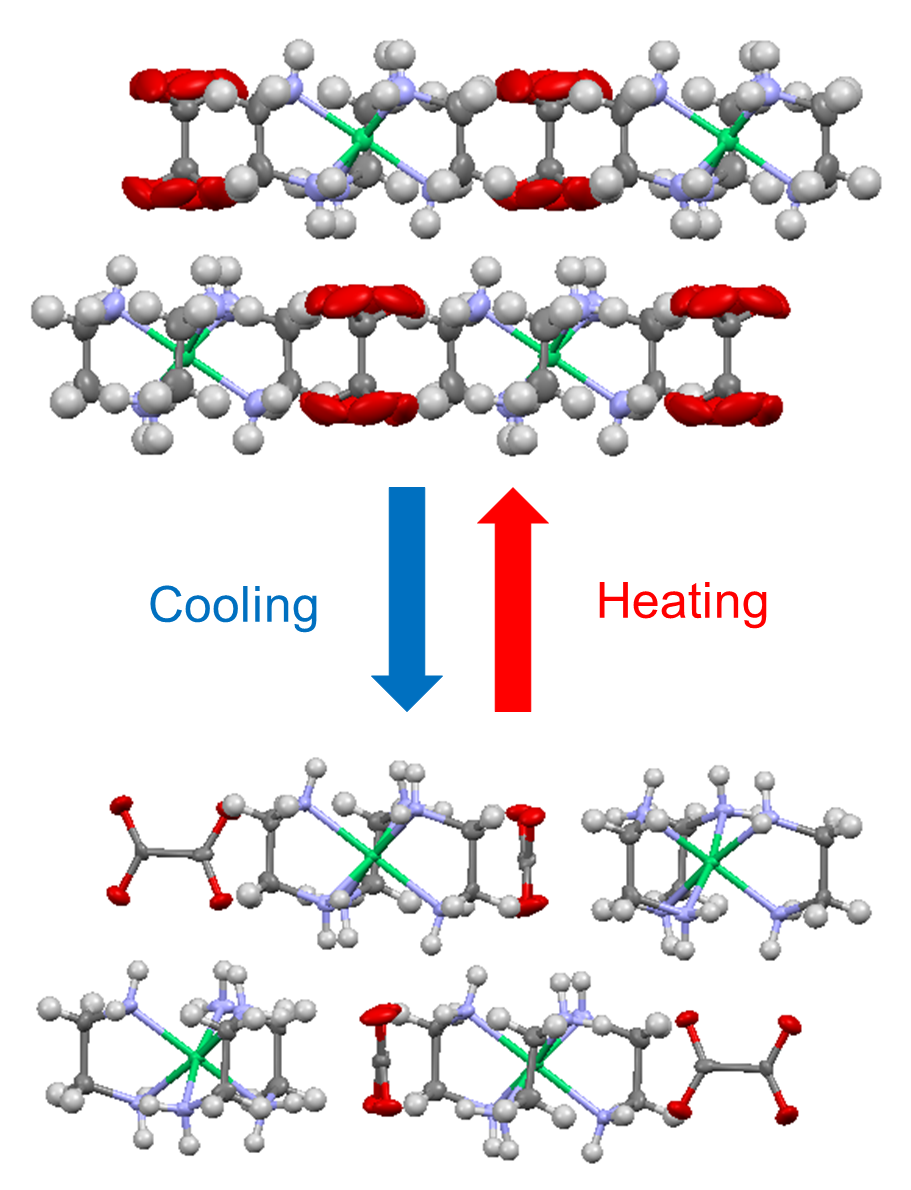
Fig. 1. Crystal structures of high-temperature (up) and low-temperature (down) phases of [NiII(en)3](ox).
Heat capacity measurement of the nickel(II) complex [NiII(en)3](ox) was carried out by adiabatic calorimetry. A large heat capacity peak was found at Ttrs = 269.0 K. Since this phase transition indicated a latent heat and a supercooled phenomenon, it is of first-order. The transition enthalpy and entropy amounted to be ΔtrsH = 6.322 ± 0.020 kJ mol-1 and ΔtrsS = 23.70 ± 0.07 J K-1 mol-1, respectively. According to S = RlnW, the number of energetically equivalent microscopic states W was estimated to be ca. 17, which suggests that many molecular motions including the rotation around the C-C bond axis of the ox2- molecule contribute to the entropy gain.

Fig. 1. Crystal structures of high-temperature (up) and low-temperature (down) phases of [NiII(en)3](ox).
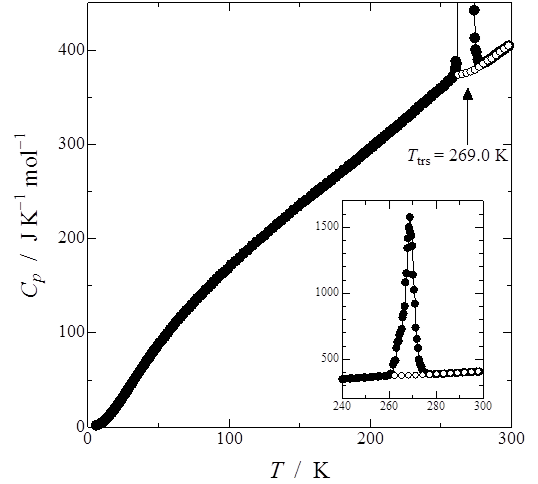
Fig. 2. Heat capacity of [NiII(en)3](ox). Inset shows heat capacity peak due to the phase transition at Ttrs = 269.0 K and supercooled phenomenon of the phase transition.