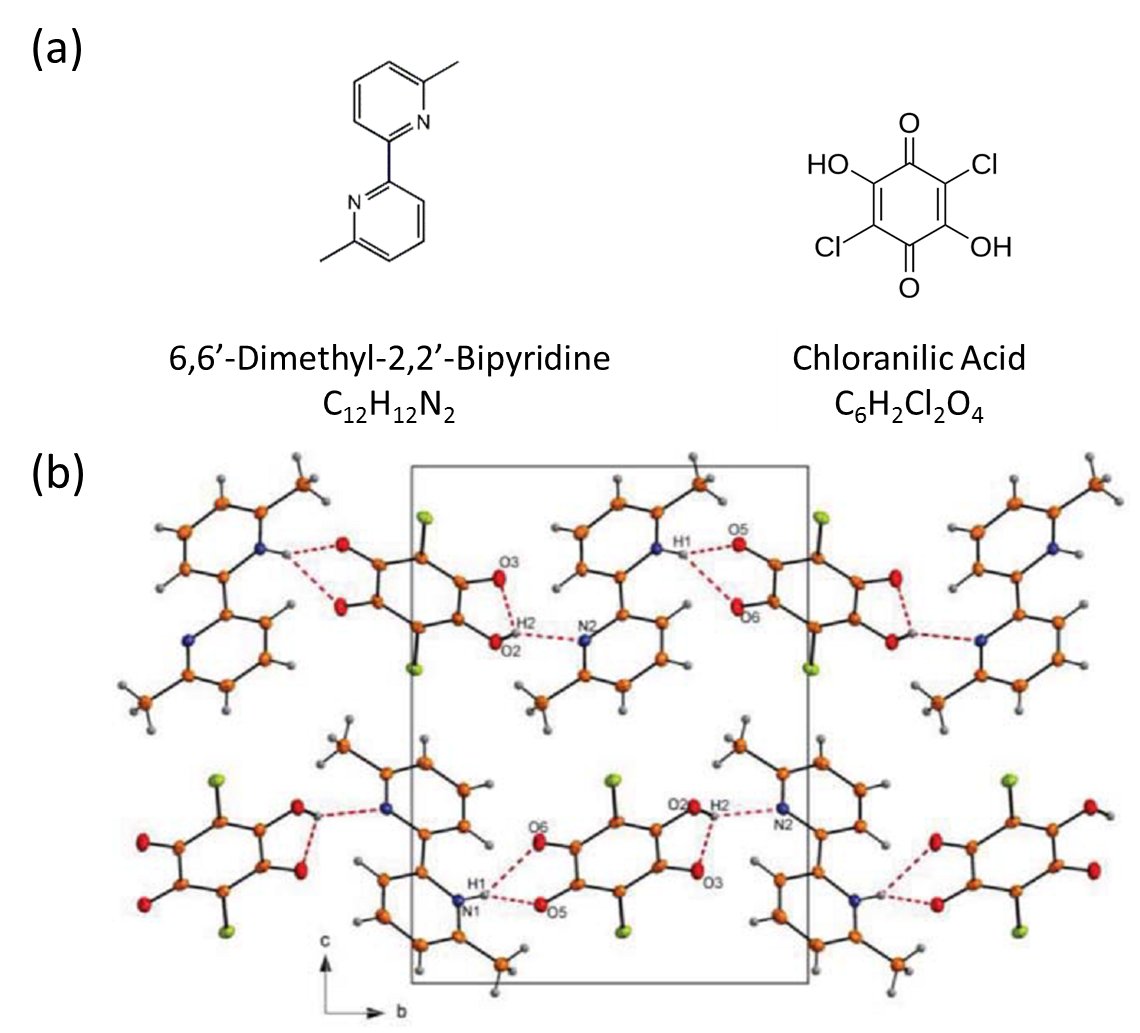
Fig. 1. (Color online) (a) Molecular structure of 6,6'-dimethyl-2,2'-bipyridine (6,6'-DMBP) and chloranilic acid (CLA). (b) Crystal structure of 6,6'-DMBP CLA along a axis.
Heat capacities of the proton-transfer complex consisting of 6,6'-dimethyl-2,2'-bipyridine and chloranilic acid, 6,6'-DMBP CLA, were measured by means of adiabatic calorimetry to investigate its thermodynamic properties in detail. In the first measurement, a first-order phase transition was observed at 376.0 K. The transition enthalpy and entropy amounted to 2.118 kJ mol−1 and 5.628 J K−1 mol−1, respectively. However, in the second or later measurement, a first-order phase transition occurred at a different temperature 372.7 K. The enthalpy and entropy changes due to this phase transition were estimated to be 1.953 kJ mol−1 and 5.237 J K−1 mol−1, respectively. Furthermore, another second-order phase transition was found at 202.7 K. The evaluated transition enthalpy and entropy were−1.530 kJ mol−1 and 7.995 J K−1 mol−1, respectively. For all the measurements, exothermic effects, which are probably due to decomposition of a small amount of the sample, were found above 350 K. Particularly, the large exothermic effect seen in the first measurement would also include stabilization from the metastable phase to the stable phase.

Fig. 1. (Color online) (a) Molecular structure of 6,6'-dimethyl-2,2'-bipyridine (6,6'-DMBP) and chloranilic acid (CLA). (b) Crystal structure of 6,6'-DMBP CLA along a axis.
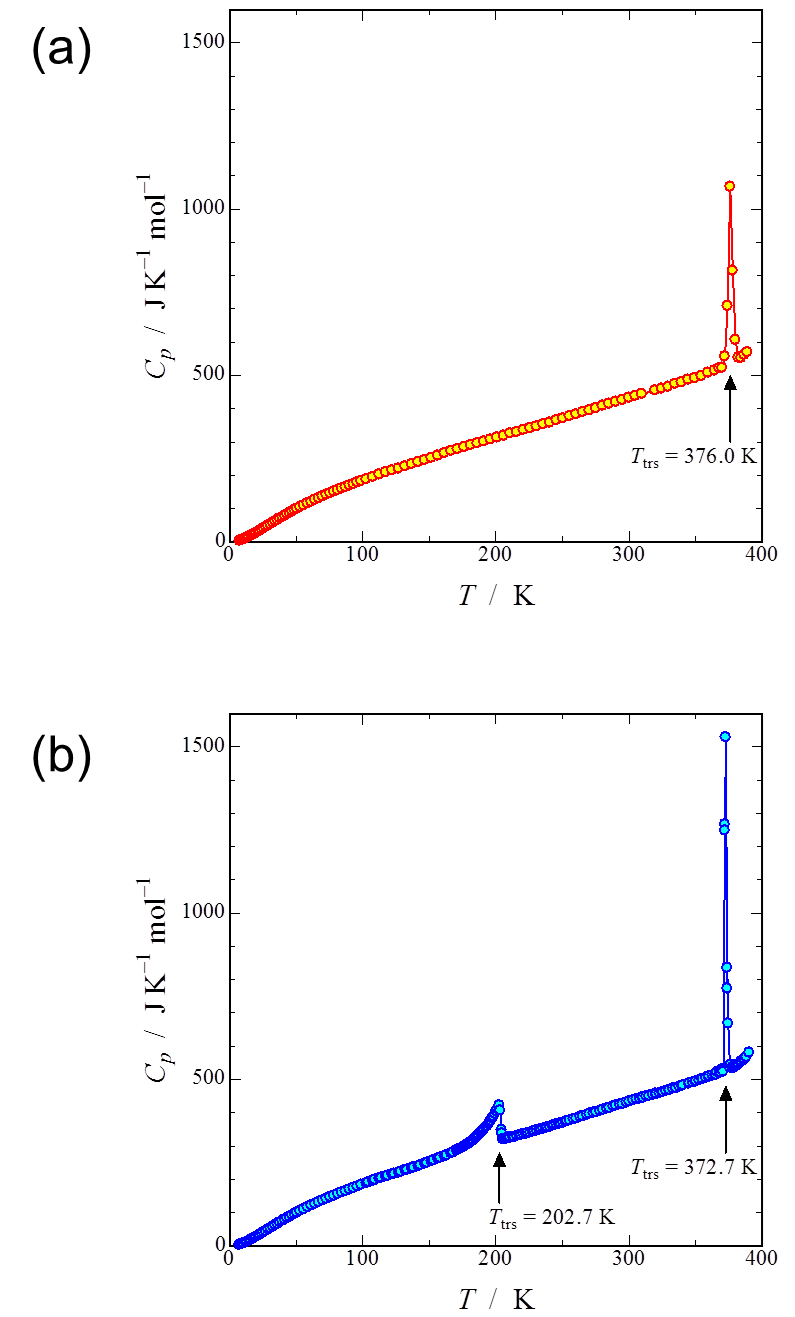
Fig. 2. (Color online) (a) Heat capacity of as-received sample. A first-order phase transition was observed at 376.0 K. (b) Heat capacity of sample which experienced the first-order phase transition once. A second-order phase transition appeared at 202.7 K and the first-order phase transition at 376.0 K shifted to 372.7 K.