 |
|
|
|
Preparation of Supramolecular Complexes with Cyclodextrin |
Preparation of Supramolecular Complexes with p-Conjugated Molecules
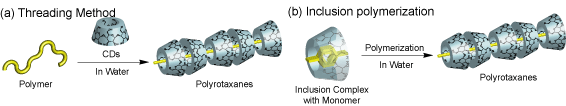
Insurated Molecular Wire Incorporating Polyrotaxane
There are two methods of synthesizing rotaxanes containing CDs in water. One method is to mix the polymer with a saturated solution of cyclodextrins. Another method is that monomers were polymerized in situ with in the cavity of cyclodextrin. p-Conjugated polymers have disadvantages in inter-chain charge-mobility and the p-p stacking interaction, both of which are essential to completing single-molecule electronic devices. However, when these polymers were the low solubility in water, it is difficult to form rotaxanes. We reported the crystal structure of the b-CDbithiophene inclusion complex and its polymerization using an oxidative initiator to form pseudo-poly(rotaxane) between b-cyclodextrin and poly(thiophene) in water.
Okada, M.; Takashima, Y.; Harada, A.
"One-Pot Synthesis of g -Cyclodextrin Polyrotaxane: Trap of g -Cyclodextrin by Photodimerization of Anthracene-Capped pseudo-Polyrotaxane"
Macromolecules, 2004, 37(19), 7075-7077.
Preparation of pseudo-Polyrotaxanes Containing Poly(thiophene)s
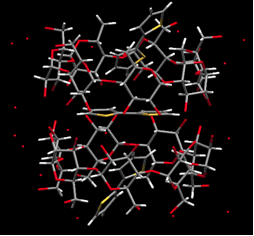
p-Conjugated polymers have attracted much interest due to their high charge-mobility along individual polymer chains. Recently, p-conjugated polymers have been used in practical applications, including light-emitting diodes, thin-film field effect transistors, photovoltaic cells, and sensors. We have prepared and determined the crystal structure of cyclodextrins inclusion complexes with various thiophenes, and the polymerization of the corresponding inclusion complexes in a selective way to obtain polyrotaxanes.
| (a) | (b) |
 |
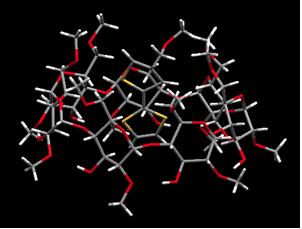 |
Crystal structure of inclusion complex. (a)2T-2,6-O-dimethyl-β-CD (b)2T-2-β-CDβ-CD |
|
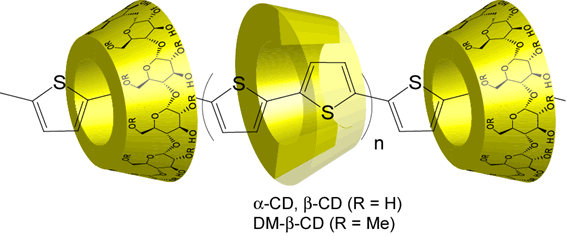
Utlizing coupling reaction, DM-β-CD poly pseudo rotaxane with polythiophene as axis was obtained.
Takashima, Y.; Sakamoto, K.; Oizumi, Y.; Yamaguchi, H.; Kamitori, S.; Harada, A.
"Complex Formation of Cyclodextrins with Various Thiophenes and Their Polymerization in Water: Preparation of pseudo-Polyrotaxanes Containing Poly(thiophene)s"
J. Incl. Phenom. Macrocycl. Chem. 2006, 56(1-2), 45-53.
Preparation and Properties of Rotaxanes Formed by Dimethyl-b-Cyclodextrin and Oligo(thiophene)s with b-Cyclodextrin Stoppers
A series of novel DM-b-CD-oligothiophene based rotaxanes have been prepared by the palladium catalyzed coupling reaction. [2]Rotaxanes and [3]rotaxanes which have various chain lengths and inclusion ratios of DM-b-CD were isolated from the crude products by using preparative reversed phase chromatography. Their photochemical properties have been investigated by UV-vis and fluorescence measurements. The inclusion ratio and the chain length of rotaxanes have been found to relate to the emission properties and the emission intensities of oligothiophene. In aqueous solutions, fluorescence quantum yields of rotaxanes were higher than those of dumbbell-shaped molecules. The increase in the fluorescence efficiency of rotaxane is caused by the suppression of intermolecular interactions, indicating the effect of insulated oligothiophene with DM-b-CD.
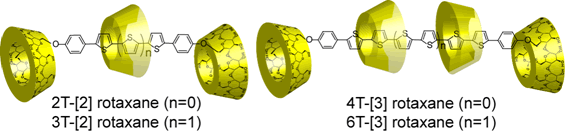
Sakamoto, K.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"Preparation and Properties of Rotaxanes Formed by Dimethyl-beta-cyclodextrin and Oligo(thiophene)s with beta-Cyclodextrin Stoppers"
J. Org. Chem. 2007, 72(2), 459-465