
Fig. 1. Molar heat capacity of CuCrP2S6.
Table 1. Phase transition temperature, enthalpy and entropy obtained for CuCrP2S6.
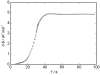
Fig. 2. The entropy of the magnetic phase transition of CuCrP2S6.
The heat capacities of CuCrP2S6 have been measured between 5 K and 350 K. Three phase transitions were observed at 30.45 K, 154.96 K and 185.92 K. The phase transition entropies based on the phonon heat capacity were 4.1, 2.7 and 6.2 J K–1 mol–1 for the three phase transitions, respectively. The phase transition occurring at 30.45 K is of a magnetic origin arising from the chromium spin and the upper two transitions are due to positional disorder of the Cu+ ions.

|
Fig. 1. Molar heat capacity of CuCrP2S6. |
|
Table 1. Phase transition temperature, enthalpy and entropy obtained for CuCrP2S6. |

|
Fig. 2. The entropy of the magnetic phase transition of CuCrP2S6. |
A high-Tc oxide superconductor YBa2Cu3Ox has been investigated by heat capacity measurements between 5 and 400 K for the samples with x=6.7, 6.9 and 7.0. All the samples showed a superconducting transition. An anomaly, which is ascribed to the glass transition due to oxygen ordering, was observed for the samples x=6.7 and 6.9. The results are consistent with those obtained by thermal expansivity measurements. Since those samples contained a compound Y2BaCuO5 as a magnetic impurity, the heat capacity of pure Y2BaCuO5 was also measured over the same temperature range to remove the magnetic contribution. The step in heat capacity at the glass transition can be explained by the one-dimensional ordering of oxygen in the lattice.
Heat capacity measurements have been performed between 5 K and 330 K for a liquid-crystalline material 5*CB [(S)-4-(2-methylbutyl)-4′-cyanobiphenyl]. The isotropic liquid can easily be undercooled to become a glass of the cholesteric phase (Tg=210.5 K). The transition from the cholesteric phase to the undercooled liquid was clearly observed at 246.6 K, being accompanied by a small enthalpy change (300 J/mol). A new phase transition was found around 100 K in the metastable solid phase, where the associated enthalpy change is about 90 J/mol. The melting consists of double peaks both for the metastable and stable phases, presumably because of the presence of the enantiomer.
We report a study of the binary phase diagram and, in particular, the glass transition behavior in glycerol/water mixtures. In the present investigation, the data for Tg also seem to extrapolate to the historically determined Tg=135 K for samples that are cooled rapidly enough to yield a homogeneous mixture. Moreover, for a partly crystallized sample, we observe a weak Tg which does vary with composition. This should be attributed to the glass transition due to the protons of ice.