Electronic Heat Capacity of Organic Superconductor
which Contains Water Molecules in the Anion Layer
Thermodynamic investigation of an organic superconductor
κ-(BEDT-TTF)2 Ag(CN)2 H2O in which the BEDT-TTF dimers are arranged in the
κ-type structure in the donor layers is
performed by the relaxation calorimetric technique at low temperatures
and under magnetic fields. A thermal anomaly related to the superconductive
phase transition was observed at 5 K. The magnitude of residual
γ* in the superconductive state is nearly 1/6
of the normal state γ value, which is larger
than those of κ-(BEDT-TTF)2 Cu(NCS)2, and
κ-(BEDT-TTF)2 Cu[N(CN)2]Br salt.
The lattice heat capacity reflected on the β-term
in the low-temperature heat capacity was found to be affected by the cooling
rate. The disorder produced in the network structure constructed by hydrogen
bond in the insulating layer is considered to give low-energy phone excitations
reflected in the heat capacity.
(by Y. Nakazawa)
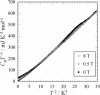
|
Fig. 1. Temperature dependence of heat capacity of κ-(BEDT-TTF)2 Ag(CN)2 H2O in a CpT −1 vs T 2 plot. A thermal anomaly related to
the superconductive transition is observed around 5 K.
|
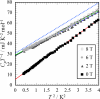
|
Fig. 2. CpT −1 vs
T 2 plot of
κ-(BEDT-TTF)2 Ag(CN)2 H2O obtained under 0 T and under magnetic fields.
The normal state electronic heat capacity coefficient γ and residual electronic heat capacity coefficient
γ* are estimated by a linear extrapolation
of the fitting lines.
|
Thermodynamic Characters of 2D-Linked Networks of Mn4 Single-Molecule Magnets under Pressures and
Magnetic fields
The magnetic properties of two-dimensional networked single-molecule magnets,
(1) [Mn4 (hmp)6 {N(CN)2}2] (ClO4)2, (2) [Mn4 (hmp)4Br2(OMe)2 {N(CN)2}2] 2THF·0.5H2O (hmp = 2-hydroxymethylpyridine, THF = tetrahydrofuran)
are studied by AC calorimetry technique under pressure up to p = 2 GPa. These compounds show peak structures in the
temperature dependence of heat capacity, which are associated with
antiferromagnetic transitions. With the increase of pressure, the Neel
temperature (TN) of (1) and
(2) show upwards and downwards sift, respectively. This result suggests that
the pressure enhances the inter-cluster magnetic interactions to increase
long-range order temperature for (1). The long-range nature in (2) is once
suppressed but enhanced under the high pressure region above 1 GPa.
(by O. Kubota & Y. Nakazawa)
|
Fig. 1. Pressure dependence of CpT −1 vs T data of
[Mn4 (hmp)6 {N(CN)2}2] (ClO4)2.
|

|
Fig. 2. Pressure dependence of CpT −1 vs T data of
[Mn4 (hmp)4Br2(OMe)2 {N(CN)2}2] 2THF·0.5H2O.
|
Thermodynamic Property of Single-Chain Magnets
under Magnetic Fields
We report results of thermodynamic measurements of series of Mn-Ni single
chain magnet (SCM), [Mn(saltmen) Ni(pao)2 (bpy)] (PF6)
(1), [Mn(3,5-Cl2saltmen) Ni(pao)2 (phen)] (PF6)
(2), and [Mn(5-Clsaltmen) Ni(pao)2 (phen)] (BPh4) (3). These samples have similar crystal structure
to each other. However, the inter-chain couplings are quite different due to
the difference of molecular structure and counter anions. Inter-chain coupling
of the (1) is strongest and that of the (3) is weakest. The heat capacity
measurement is performed by the thermal relaxation technique under magnetic
fields. (1) shows peak structure corresponding to anti-ferromagnetic transition
due to inter-chain coupling around 11 K. This peak is suppressed by magnetic
field. On the other hands, (2) shows no peak structure in the whole temperature
region (5 K – 30 K). However large magnetic fields dependence of
heat capacity curve is observed in (2) around 12 K. Similar tendency
is observed more clearly in (3).
(by S. Yamashita & Y. Nakazawa)
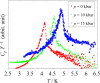
|
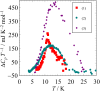
Fig. 1. Heat capacity of [Mn(saltmen) Ni(pao)2 (bpy)] (PF6) (1), [Mn(3,5-Cl2saltmen) Ni(pao)2 (phen)] (PF6)
(2), and [Mn(5-Clsaltmen) Ni(pao)2 (phen)] (BPh4) (3) under magnetic field.
|
|
Fig. 2. Differences of heat capacities under 0 T and several
high magnetic fields; [Mn(saltmen) Ni(pao)2 (bpy)] (PF6) (1) (0 T – 8 T), [Mn(3,5-Cl2saltmen) Ni(pao)2 (phen)] (PF6)
(2) (0 T – 8 T), [Mn(5-Clsaltmen) Ni(pao)2 (phen)] (BPh4) (3) (0 T – 12 T).
|
Magnetic Property of Cu7 Complex
at Extremely-Low Temperatures
We report results of the low-temperature thermodynamic measurement of
heptacopper complexes, [Cu7 (μ3-Cl)2(μ3-OH)6 (D-pen-disulfide)3]. This complex consists of two cubane units and forms
an inversed pyramidal structure in which 7 Cu ions are included. The constitutive
copper cations are divalent. The ground state of the magnetic complex is studied
by theoretical calculation. The heat capacity measurement is perfomed by thermal
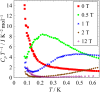
relaxation method under magnetic fields and at low temperatures. There is no
magnetic transition below 10 K. However, broad peak structure seems to be a
schottky heat capacity judging from data under magnetic field. From the value of
the peak (4 J K−1 mol−1) and peak temperature under magnetic fields,
the ground state of this system is considered to be S = 1/2 state.
(by N. Tokoro & S. Yamashita & Y. Nakazawa)
|
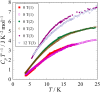
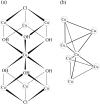
Fig. 1. (a) Schematic representations of core structures of the
[Cu7 (μ3-Cl)2(μ3-OH)6 (D-pen-disulfide)3].
(b) Schematic illustrations for spin cages of [Cu7 (μ3-Cl)2(μ3-OH)6 (D-pen-disulfide)3].
|
|
Fig. 2. Temperature dependence of heat capacity of [Cu7 (μ3-Cl)2(μ3-OH)6 (D-pen-disulfide)3]
under magnetic fields.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.







