Magnetic Heat Capacity and Magnetic Structure of the
Organic Magnet PhBABI
Heat capacities of the hydrogen-bonded organic magnet PhBABI were measured by
adiabatic calorimetry and relaxation method under magnetic fields. No thermal
anomaly was detected at high temperatures. On the other hand, a small thermal
anomaly was observed below 3 K. The derived magnetic heat capacities exhibited
a hump around 15 K. Magnetic entropy was evaluated to be 5.88 or 5.75
J K−1 mol−1, which agrees well with the expected value for
S = 1/2 spin systems Rln2 (=
5.76 J K−1 mol−1). The hump around 15 K was reproduced well by both
S = 1/2 spin ladder and bilayer models.
(by Y. Miyazaki)
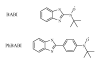
|
Fig. 1. Molecular structures of BABI and PhBABI.
|

|
Fig. 2. Heat capacities (upper) and magnetic heat capacities (lower) of
PhBABI under magnetic fields. Solid curve indicates the lattice heat capacity.
Broken curve shows the spin wave heat capacity with an energy gap.
|

|
Fig. 3. Comparison between zero-field magnetic heat capacity of PhBABI
and magnetic models. Purple, black, green, red, and blue curves represent
S = 1/2 1D chain, 2D square planar, singlet-triplet,
bilayer, and spin ladder models, respectively.
|
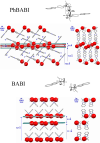
|
Fig. 4. Packings of major spin density sites and closest contacts between
nitroxides in PhBABI (upper) and BABI (lower).
|
Heat Capacity and Magnetic Phase Transition of the One-Dimensional
Organic Ferromagnet F4BImNN
Heat capacities of the hydrogen-bonded one-dimensional organic ferromagnet
F4BImNN were measured by adiabatic calorimetry and relaxation method under
magnetic fields. A broad non-magnetic thermal anomaly was detected around
120 K. A heat capacity peak due to magnetic phase transition was found at
0.72 K. Magnetic field dependence of the magnetic phase transition temperature
revealed that the observed magnetic phase transition is antiferromagnetic.
(by Y. Miyazaki)
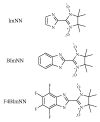
|
Fig. 1. Molecular structures of ImNN, BImNN, and F4BImNN.
|

|
Fig. 2. Heat capacity (upper) and heat capacity divided by temperature
(lower) of F4BImNN by adiabatic calorimetry.
|

|
Fig. 3. Heat capacities of F4BImNN under magnetic fields.
|

|
Fig. 4. Magnetic field dependence of antiferromagnetic phase transition
temperature of F4BImNN. Solid curve indicates the fitting theoretical curve.
|
Heat Capacity and Magnetic Phase Transition of the Molecule-Based
Magnet DOT• + · FeIII Cl4−
Heat capacities of 2,2′:6′,2″-dioxytriphenylamine radical
cation DOT• + (S =
1/2) and FeIII Cl4− (S = 5/2)
salt DOT• + · FeIII Cl4− were measured by adiabatic
calorimetry and relaxation method under magnetic fields. A broad non-magnetic
thermal anomaly was found around 250 K. A large heat capacity peak was observed
at 6.82 K. This peak is due to antiferromagnetic phase transition from the
magnetic field dependence of the peak temperature. A tiny heat capacity peak
due to antiferromagnetic phase transition was also detected at 0.62 K, which
would come from a small amount of imperfect crystals. The estimated magnetic
entropy 13.4 J K−1 mol−1 is close to Rln5 (= 13.4
J K−1 mol−1) for S = 2 spin systems
rather than Rln(2×6) (= 20.7 J K−1 mol−1) for
S = 1/2 and 5/2 spin systems. This suggests that the
spins of DOT• + (S
= 1/2) and FeCl4−
(S = 5/2) are coupled with a strong antiferromagnetic
interaction, giving rise to S = 2 resultant spin to
exhibit the antiferromagnetic phase transition at 6.82 K. From the ratio of
the magnetic entropy above the transition temperature to the whole magnetic
entropy, DOT• + · FeCl4− would have a three-dimensional face-centered or
body-centered cubic magnetic structure.
(by X.-Z. Lan & Y. Miyazaki)
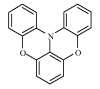
|
Fig. 1. Molecular structure of DOT.
|

|
Fig. 2. Heat capacity of DOT• + · FeCl4− by adiabatic calorimetry.
|

|
Fig. 3. Heat capacities of DOT• + · FeCl4− under magnetic fields. Solid curve indicates
the lattice heat capacity. For the sake of clarity, the heat capacities
except for the zero-field heat capacity are shifted upwards.
|

|
Fig. 4. Magnetic heat capacities of DOT• + · FeCl4− under magnetic fields. For the sake of clarity,
the magnetic heat capacities except for the zero-field
magnetic heat capacity are shifted upwards.
|
Heat Capacity and Size Dependence of
Ni(OH)2 Monolayer Nanocluster
in Amorphous SiO2
Heat capacities of Ni(OH)2 monolayer nanoclusters
(Ni-MNC) were measured from 1.85 K to 100 K by relaxation method.
All the Ni-MNCs exhibited a broad heat capacity anomaly around 20 K.
The magnetic entropies for the Ni-MNCs are lower than the expected value
Rln3 for S = 1
spin systems. Furthermore, the magnetic thermal anomaly and thus
the magnetic entropies for the Ni-MNCs depend on the synthetic condition,
which might be attributed to the difference of the Ni-MNC size.
(by T. Maruoka & Y. Miyazaki)
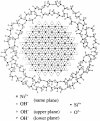
|
Fig. 1. Structure of Ni-MNC in amorphous SiO2.
|
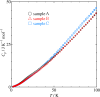
|
Fig. 2. Apparent heat capacities of Ni-MNCs in samples A, B, and C.
|
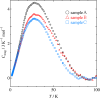
|
Fig. 3. Magnetic heat capacities of Ni-MNCs in samples A, B, and C.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.














