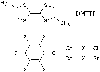
Fig. 1. Molecular structures of DMTTF, CA, and BA.
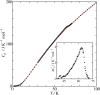
Fig. 2. Heat capacity of DMTTF-CA. Inset shows its excess heat capacity.
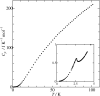
Fig. 3. Heat capacity of DMTTF-BA.
Heat capacities of the mixed-stack organic charge transfer complexes composed of 4,4′-dimethyltetrathiafulvalene (DMTTF) and tetrahalo-p-benzoquinones have been measured in the temperature range between 0.35 and 100 K by relaxation method. In DMTTF-p-chloranil (DMTTF-CA), a small and broad peak associated with the neutral-ionic phase transition (N-I transition) was observed at 63 K. The transition enthalpy and entropy were 47.3 J mol−1 and 0.809 J K−1 mol−1, respectively, which suggest that this N-I transition is displacive type. On the other hand, DMTTF-p-bromanil (DMTTF-BA) exhibited a small peak at 2.6 K. The magnetic-field dependence of the transition temperature revealed that this transition is not a magnetic phase transition. This transition is probably spin-Peierls transition.
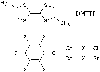
|
Fig. 1. Molecular structures of DMTTF, CA, and BA. |

|
Fig. 2. Heat capacity of DMTTF-CA. Inset shows its excess heat capacity. |

|
Fig. 3. Heat capacity of DMTTF-BA. |
Heat capacity measurements have been performed for the crystalline lithium acetate dihydrate and its methyl-(partially)-deuterated analogs between 0.35 K and 300 K. We found various phase transitions for the deuterated compounds at low temperatures, which can be explained by orientational ordering of methyl groups, where two neighboring methyl groups rotate together. We also found a phase transition at 176 K for all the compounds, which is presumably due to the water molecules in the crystal.
Heat capacity measurements were made for 4-methylpyridine and its deuterated analogs (C5H4N-CH3, C5H4N-CH2D, C5H4N-CHD2, and C5H4N-CD3). C5H4N-CD3 exhibits a λ-type phase transition at 8.6 K, whereas C5H4N-CH2D and C5H4N-CHD2 show a phase transition at 5.2 K and 7.5 K respectively, with a broad hump in heat capacity. While C5H4N-CH3 doesn't show such a transition, it shows a thermal relaxation around 10 K which may be attributed to the nuclear spin conversion of the protons in the methyl groups. The transition entropy is ΔS = 3.0 J K−1 mol−1 for C5H4N-CD3, ΔS = 7.9 J K−1 mol−1 for C5H4N-CH2D, and ΔS = 10.1 J K−1 mol−1 for C5H4N-CHD2. The orientational order-disorder model, where two methyl groups face to each other, explains the transition entropy of C5H4N-CD3. For C5H4N-CH2D and C5H4N-CHD2, however, the expected value is significantly larger than the observed ones, indicating that the orientations of the methyl groups are not completely settled even at the lowest temperature. There still remains quantum picture for those partially deuterated analogs.
Heat capacity measurements were made for 5*CB [(S)-4-(2-methylbutyl)-4′-cyanobiphenyl] and 8*OCB [(S)-4-(1-methylheptyloxy)-4′-cyanobiphenyl] between 0.35 K and 20 K. For 5*CB, excess heat capacities were observed in the glass of the liquid crystal and the glassy metastable crystal below 1 K, while the stable crystal did not show such a tendency. The equation Cp = c1T + c3T 3 was used to fit the heat capacities obtained below 1 K for each phase. The Debye temperatures were calculated from the coefficient c3. The Debye temperature of the glassy metastable crystal is higher than that of the stable crystal indicating that the metastable crystal has larger density than that of the stable crystal. For 8*OCB, interestingly, the stable crystal (C1) has excess heat capacity below 1 K, and the heat capacity of the glassy metastable crystal (C2) below 1 K seems rather normal.