Low-Temperature Heat Capacity of
Partially Crystallized Mixtures of
the Binary System of Water and Glycerol
In the binary system of water and glycerol, the crystallization behavior of
the pure components, namely the proneness to crystallization of water as well
as the glass-forming tendency of glycerol, is fully displayed in the composite
system. For mixtures rich in one of the components, either partial
crystallization (water-rich mixtures) or formation of a homogeneous glass
(glycerol-rich mixtures) is observed. When water partially crystallizes out of
a mixture of intermediate concentration, both regular hexagonal ice and a
“cubic (Ic)” ice with some two-dimensional order can be obtained
selecting the appropriate annealing temperature. Moreover, it has been
suggested that parallel to the partial crystallization of water, a remaining
glycerol-rich homogeneous phase of fixed concentration is always formed. The
formation of a remaining glycerol-rich homogeneous phase of about 76% glycerol
mass fraction during partial crystallization was experimentally confirmed.
(by O. Camacho & A. Inaba)

|
Fig. 1. Neutron diffraction patterns obtained from glycerol water (D2O) mixture at 55% (in mass). The cubic ice with some 2D
feature appeared at 190 K and it transformed into hexagonal ice at 220 K.
|

|
Fig. 2. The glass (Tg)
and melting (Tm)
transition temperatures as a function of glycerol content.
Only the relevant portion is displayed.
|

|
Fig. 3. Comparison of the heat capacities obtained for an aqueous
solution of glycerol (60% in mass). The homogeneous glass has a higher
heat capacity than that of the ice crystallized mixtures.
|
Heat Capacity of
Alkaline Metal Intercalated C60 Phases
Heat capacity measurements have been performed between 0.4 K and 350 K for
the alkali doped C60 phases, Li4C60 and Na4C60, as well as the pressure
polymerized C60. According to the structural and
spectroscopic studies, the C60 molecules are
2-dimensionally polymerized in the lattice. We compare the low temperature
heat capacity to investigate the polymer bond type. Li4C60 shows the similar heat
capacity to that for the pressure polymerized C60,
whereas Na4C60 shows
rather large heat capacity.
(by A. Inaba)
|
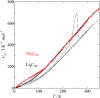
Fig. 1. Molar heat capacity of Li4C60 and Na4C60, together with 1D and 2D pressure polymerized C60 and pristine C60.
|
|
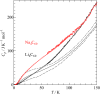
Fig. 2. Low-temperature heat capacity of
Li4C60 and
Na4C60.
|
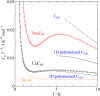
|
Fig. 3. Low-temperature heat capacity of Li4C60 and Na4C60. The former shows similar
heat capacity as in 2D pressure polymerized C60,
the latter shows rather large contribution.
|
Heat Capacity and Phase Transition of Cyclohexane-Solvated
C60 Crystal
Heat capacities of cyclohexane-solvated C60 crystal
in cyclohexane solution were measured by adiabatic calorimetry. A large heat
capacity peak due to the phase transition of
C60 (C6H12)13 crystal was observed at
119.7 K and the phase transition and fusion of cyclohexane were found at
186.2 K and 279.8 K, respectively. Furthermore,
C60 (C6H12)13 crystal changed into
C60 (C6H12)2 crystal at 342 K. The
transition enthalpy and entropy of
C60 (C6H12)13 crystal were evaluated to be
24.69 kJ mol−1 and 206.6 J K−1 mol−1,
respectively. On the other hand,
C60 (C6H12)2 crystal obtained by rapid
cooling exhibited double peaks due to the phase transition at 131.0 K and
152.2 K. The transition enthalpy and entropy of
C60 (C6H12)2 crystal were 4.549
kJ mol−1 and 31.83 J K−1 mol−1,
respectively.
(by Y. Miyazaki)
|
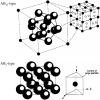
Fig. 1. Crystal structures of AB13- and AB2-types.
|

|
Fig. 2. Heat capacities of C60 (C6H12)13
(upper) and C60 (C6H12)2
(lower) crystals in cyclohexane solution.
|

|
Fig. 3. Heat capacities of C60 (C6H12)13
(upper) and C60 (C6H12)2
(lower) crystals.
|
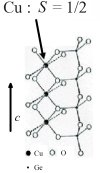
Thermal Conductivity of
a Low-Dimensional Spin System CuGeO3
Thermal conductivity via electronic degrees of freedom has been a valuable
tool to study exotic electron systems such as high-Tc superconductors.
In our present experiments, thermal conductivity is measured for a series of
high-quality single crystals of CuGeO3 and its
impurity-doped compounds, as a representative of one-dimensional spin systems.
It is demonstrated that spin excitations carry an exceptionally large amount of
heat in the one-dimensional quantum magnet, indicating their excellent spatial
coherence like phonons and electrons in metals. The results also indicate that
thermal conductivity measurement is a useful probe to in studying exotic
spin-related properties of strongly-correlated electron systems in general.
(by J. Takeya)
|
Fig. 1. Crystal structure of CuGeO3.
|

|
Fig. 2. Thermal conductivity of Cu1−xMgxGeO3 in the direction
of the spin chain with various Mg concentraiton x.
|

|
Fig. 3. Thermal conductivity of Cu1−xMgxGeO3 as function of
x in the chain direction.
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.











