Schottky Type Anomaly in Heat Capacity
Caused by Partial Deuteration
of Methyl Group in 2,6-Dichlorotoluene
Heat capacity measurements were made for 2,6-dichlorotoluene
(C6H3Cl2-CH3)
and its methyl-deuterated analogs,
C6H3Cl2-CH2D,
C6H3Cl2-CHD2,
C6H3Cl2-CD3,
between 0.35 K and 300 K by adiabatic calorimetry and relaxation calorimetry.
A Schottky type anomaly was found for the two partially deuterated analogs,
C6H3Cl2-CH2D and
C6H3Cl2-CHD2, below 40 K.
It is explained by an energy scheme involving three levels,
g1/g0 =
g2/g0 = 1.
The spacing from the ground state is 267 μeV and 2950 μeV for
C6H3Cl2-CH2D
and 2400 μeV and 2700 μeV for
C6H3Cl2-CHD2, respectively.
The excess entropy amounts to Rln3 (= 9.13
J K−1 mol−1)
which is due to the symmetry breaking of methyl group by partial deuteration.
(by H. Suzuki & A. Inaba)
|
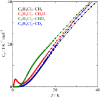
Fig. 1.
Low temperature heat capacity of 2,6-dichrolotoluenes. The partially
deuterated compounds (–CH2D and
–CHD2) showed a broad anomaly
in heat capacity, whereas the fully protonated and
fully deuterated compounds showed no anomaly.
|

|
Fig. 2.
Excess heat capacity of partially deuterated 2,6-dichorolotoluenes
(–CH2D and
–CHD2). Solid curves indicate
the Schottky type heat capacities; the energy schemes of the models
are shown schematically in the figure.
|
|
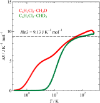
Fig. 3.
Excess entropy of partially deuterated 2,6-dichorolotoluenes
(–CH2D and
–CHD2). Dashed line indicates the value of
ΔS = Rln3 (= 9.13
J K−1 mol−1).
|
Copyright © Research Center for Structural Thermodynamics,
Graduate School of Science, Osaka University. All rights reserved.


