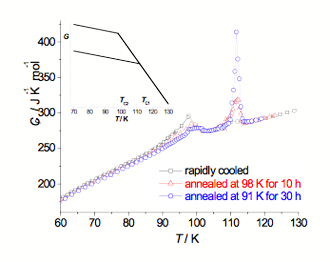
 Fig. 1. Molar heat capacity curves registered for [Co(NH3)6](ClO4)3. Inset: schematic diagram of phase relation.
Fig. 1. Molar heat capacity curves registered for [Co(NH3)6](ClO4)3. Inset: schematic diagram of phase relation.
Crystal structure of the titled compounds at room temperature consists of octahedral M(NH3)6 ions with nearly freely rotating NH3 ligands around the threefold axis, and of tetrahedral XY4 ions connected with NH3 groups by weak hydrogen bonds. In fact, all the compounds studied exhibit orientational dynamical disorder of NH3 ligands, as well as of M(NH3)6 cations and XY4 anions, across a wide temperature range, and therefore, belong to so-called orientationally dynamically disordered crystals. The phase behavior of these compounds was studied previously using DSC and NMR methods. In our resent studies, we focused on the phase behavior at lower temperature range below 120 K.
 Fig. 1. Molar heat capacity curves registered for [Co(NH3)6](ClO4)3. Inset: schematic diagram of phase relation.
Fig. 1. Molar heat capacity curves registered for [Co(NH3)6](ClO4)3. Inset: schematic diagram of phase relation.
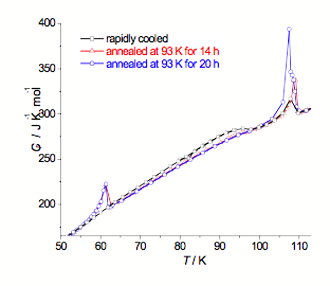 Fig. 2. Molar heat capacity curves registered for [Cr(NH3)6](BF4)3
Fig. 2. Molar heat capacity curves registered for [Cr(NH3)6](BF4)3
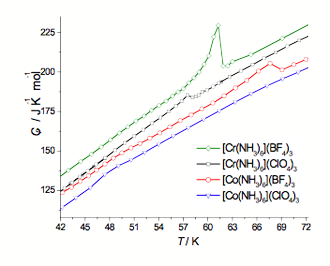 Fig. 3. Molar heat capacity curves registered for all four compounds in the temperature range between 42 and 72 K.
Fig. 3. Molar heat capacity curves registered for all four compounds in the temperature range between 42 and 72 K.
Solid polymorphism of four compounds was studied using adiabatic calorimetry (AdC) (5 – 300 K), Fourier transform middle infrared absorption spectroscopy (FT–MIR) (15 – 300 K), and X–ray single crystal diffraction (XRSCD) (90 – 300 K). Fig. 1 shows molar heat capacity Cp curves for [Co(NH3)6](ClO4)3, obtained under three different experimental conditions. When the sample was rapidly cooled from room temperature and then measured, two small peaks at 98 K and 110 K in Cp curve were registered and some small heat evolution was also detected at ∼98 K. With annealing the sample at this temperature, the peak at 98 K became smaller whereas the peak at 110 K became bigger. However, even after 30 hours of annealing we still observed some heat evolution, which indicated slow stabilization to another phase. The transition observed at TC1 = 111.8 K (for annealed sample) is thus a transition between stable phases whereas the transition at TC2 = 97.6 K (for quenched sample) is a transition between metastable phases.
Similar thermal behavior was detected for [Cr(NH3)6](BF4)3 (see Fig. 2). When the sample was rapidly cooled and then measured, some small heat evolution was detected at ~95 K. There is one transition observed at TC1 = 107.7 K in Cp curve and also one very broad anomaly with maximum at ∼96 K, which can be associated with another phase transition between metastable phases or with some spontaneous stabilization occurring within the sample. Annealing the sample at 93 K decreased the heat evolution, which vanished completely after annealing the sample for 20 hours. Two phase transitions were detected at TC1 = 107.5 K and TC2 = 61.3 K for annealed sample.
Molar heat capacity curves obtained for all four compounds in the temperature range between 42 K and 72 K are presented in Fig. 3. The results of [Cr(NH3)6](BF4)3 and [Co(NH3)6](ClO4)3 are for annealed samples. [Cr(NH3)6](BF4)3 and [Co(NH3)6](ClO4)3 do not exhibit any metastable phases and undergo only one phase transition in low temperature range, at TC = 57.5 K for the chromium compound and at TC = 67.6 K for the cobalt compound. Interestingly, these phase transition temperatures are close to the one obtained for [Cr(NH3)6](ClO4)3 at TC3 = 61.3 K after annealing but [Co(NH3)6](ClO4)3 does not exhibit any phase transition in this temperature range.
The phase transitions observed in all four compounds, which were rapidly cooled before measurements, are most likely connected with significant slowing down of rotation of NH3 groups, confirmed by temperature analysis of full width at half maximum (FWHM) of the infrared absorption band connected with ρr(NH3) F1u mode. Additionally, the more complex polymorphism observed in[Co(NH3)6](ClO4)3 and [Cr(NH3)6](BF4)3 can be connected with distortion of coordination cations M(NH3)63+ in these compounds. Because of this distortion, not all distances between metal and nitrogen are equivalent, which may cause NH3 ligands to rotate with different speeds depending on the position in crystal lattice. So far, we have discovered such distortion of Co(NH3)63+ in [Co(NH3)6](ClO4)3 across the whole investigated temperature range between 90 and 300 K, using XRSCD method. Concurrently, we have not observed this distortion in [Cr(NH3)6](BF4)3. The similar investigations for both chromium compounds are presently in progress.
N. Górska, A. Inaba, and E. Mikuli, the 21st IUPAC International Conference on Chemical Thermodynamics (Tsukuba), IM-2201-1720 (2010).
Copyright © Research Center for Structural Thermodynamics, Graduate School of Science, Osaka University. All rights reserved.