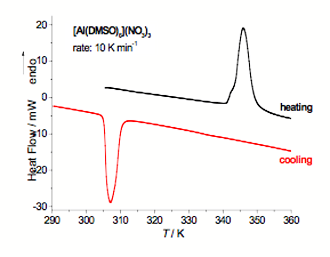
 Fig. 1 DSC curves registered during heating and subsequent cooling for [Al(DMSO)6](NO3)3.
Fig. 1 DSC curves registered during heating and subsequent cooling for [Al(DMSO)6](NO3)3.Dimethyl sulfoxide (DMSO = (CH3)2SO) is well known molecule in coordination chemistry because it can be easily coordinated to central metal through oxygen or sulfur atom. Using complementary physicochemical methods one can analyze the degree of disorder of DMSO molecules as well as the speed of rotation of CH3 groups being part of DMSO in coordination compounds, at different temperatures. The phase behavior of the [M(DMSO)6](XY4)2 compounds, where M = Mg, Zn, Co, Cd, Cu, Mn, and XY4− = ClO4− or BF4− has been studied before and is characterized by rich polymorphism.
Our present interest is on the DMSO compounds with NO3− ions with trigonal planar symmetry. Thermal behavior of two compounds: [Al(DMSO)6](NO3)3 and [Sr(DMSO)4(NO3)2] was studied using differential scanning calorimetry (DSC) (100 – 380 K), thermogravimetric (TG) and differential thermal analysis (DTA) (300 – 850 K), Fourier transform middle infrared absorption spectroscopy (FT–MIR) (20 – 380 K), and X–ray single crystal diffraction (XRSCD) (90 – 300 K).
Depending on the type of central metal, different number of DMSO molecules can be attached in coordination sphere. The aluminum compound is an example of six coordinated structure. It contains Al(DMSO)63+ ions of octahedral symmetry and NO3− ions connected with CH3 groups with weak hydrogen bonds beyond the coordination sphere. From DSC measurements, one solid-solid transition was observed for this complex at 343.2 K (on heating) and 310.8 K (on cooling) (see Fig. 1). The large thermal hysteresis indicates that the transition is of the first order. From a splitting of some infrared bands of the FT–MIR spectra, registered at temperatures below the transition, it was deduced that the transition is associated with the lowering of crystal structure symmetry. Additionally, the temperature analysis of full width at half maximum of ρr(CH3) mode revealed significant increase in the speed of rotation of CH3 groups above this transition. The phase transition temperature and the transition entropy (ΔS = 75 J K−1 mol−1) are comparable with the values obtained for the [M(DMSO)6](XY4)2 compounds. However, it is interesting to note that this compound does not show any complicated polymorphism, which was discovered in the [M(DMSO)6](XY4)2 compounds.
 Fig. 1 DSC curves registered during heating and subsequent cooling for [Al(DMSO)6](NO3)3.
Fig. 1 DSC curves registered during heating and subsequent cooling for [Al(DMSO)6](NO3)3.
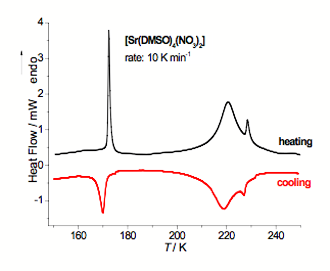 Fig. 2 DSC curves registered during cooling and subsequent heating for [Sr(DMSO)4(NO3)2].
Fig. 2 DSC curves registered during cooling and subsequent heating for [Sr(DMSO)4(NO3)2].
The strontium compound is an example of eight coordinated structure. All four DMSO ligands as well as two NO3− groups are coordinated to a central metal through oxygen atoms. The NO3− groups act as bidentate ligands. The different crystal structure of compound investigated has a big influence on its thermal behavior. The compound undergoes two solid-solid transitions at TC1 = 221.8 K and TC2 = 174.8 K (on heating) (see Fig. 2). The XRSCD results show that in the lowest temperature phase (below TC2) the crystal structure consists of all completely ordered DMSO groups. In the intermediate phase two types of the [Sr(DMSO)4(NO3)2] units exist. One type contains all DMSO molecules ordered. Another one contains two out of four DMSO molecules disordered with sulfur atoms occupying two possible positions with major occupancy 65%, and with positions of methyl carbons remaining unchanged. The high temperature phase (above TC1) is characterized by even higher degree of dynamical disorder of DMSO groups.
The investigated complexes differ also in thermal stability, which was studied using TG/DTA methods. [Al(DMSO)6](NO3)3 is stable up to 400 K and decomposes in the temperature range between 400 and 850 K, liberating all six DMSO ligands in one main step. [Sr(DMSO)4(NO3)2] melts at 346 K and decomposes in the temperature range between 370 and 1120 K, liberating gradually DMSO ligands in two main steps.
N. Górska, A. Inaba, and A. Migdał-Mikuli, the 2nd International Symposium on Structural Thermodynamics (Toyonaka), O-9 (2010).