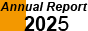About Research
Heat Capacity Analysis for Magnetic Materials
Heat capacity measurement is a quite effective method to analyze molecular magnetic substances having fine structures of energy levels. Fe42 cyanide-bridged nanocage shown above has 100 trillion (618) of magnetic states per one molecule through magnetic interactions between Fe(III) ions. The magnetic heat capacities of Fe42 at low temperature are reproducible by using a simple mean field approximation and the total entropies are consistent with the expected value (18R ln6 = 268 J K-1 mol-1), indicating that heat capacity measurements enable us to observe the total number of the magnetic states directly.
Nature Commun. 6, 8810 (1-7) (2015)
Motions of Loosely Bound Atoms and Molecules in Crystal
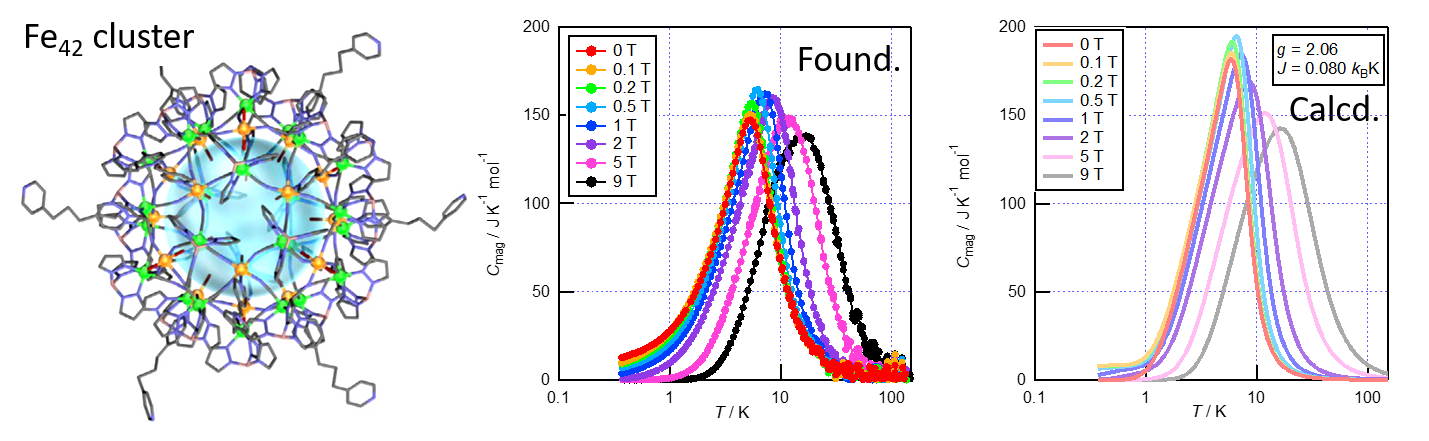
In general atoms and molecules are arranged in highly ordered structure in crystal. However some crystals have loosely bound atoms or molecules whose positions or orientations are dynamically disordered. The weak interactions between those atoms (molecules) sometimes bring about dramatic changes in structure, dynamics and thermodynamic properties with changing temperature. The above figures show the THz spectrum and heat capacity of Li+ ions encapsulated in fullerenes C60. It was revealed that the positions of Li+ ions are changed with temperature.
Phys. Chem. Chem. Phys. 18, 31384-31387 (2016).
Self-Assembly of Organic Molecules
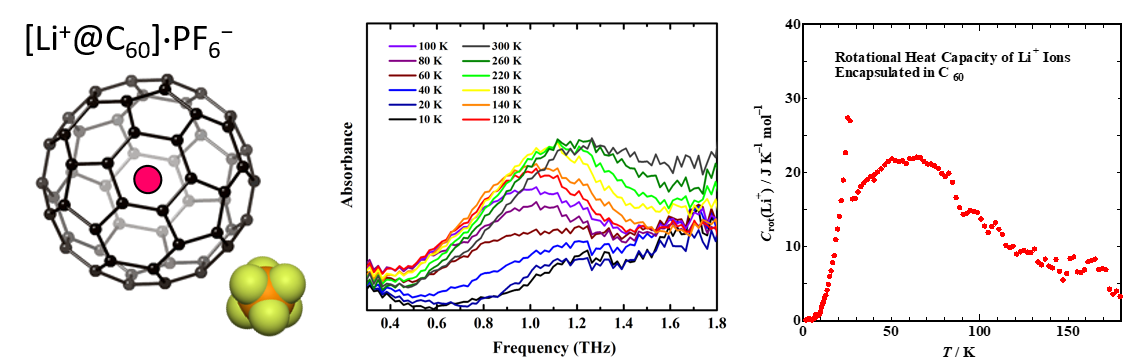
We are interested in the self-assembly of organic molecules as a theme of natural science. Our aim is to get the insight on the molecular behavior on the solid surface by scanning probe microscopy (STM). Furthermore, we examine the effect of the external stimulations on the self-assembly, such as optical irradiation, heating, and air oxidation.
Langmuir 35, 2123-2128 (2019).
Thermodynamic Approach to the Development of Living Organisms
Thermodynamics aims to interpret development in terms of entropy. Fundamental laws of thermodynamics are general and do not depend on the characteristics of working substances. Therefore, they show the general laws which dominate elementary particles, space, living organisms, and even societies. Chemical thermodynamics completely interprets all the chemical phenomena, so it is certainly the most established science. On the other hand, the higher hierarchy, including living organisms and societies, gives us huge uncultivated fields for thermodynamics, in which mesoscopic/macroscopic interaction time grows close to observation time and then they locate far from equilibrium. Thermodynamic research on development of living organisms does not take care about the details of chemistry of metabolism, but try to catch them as a whole. Calorimetry doubtlessly provides us the most effective and reliable method in such researches.
Phys. Biol. 11, 046008 (1-12) (2014)
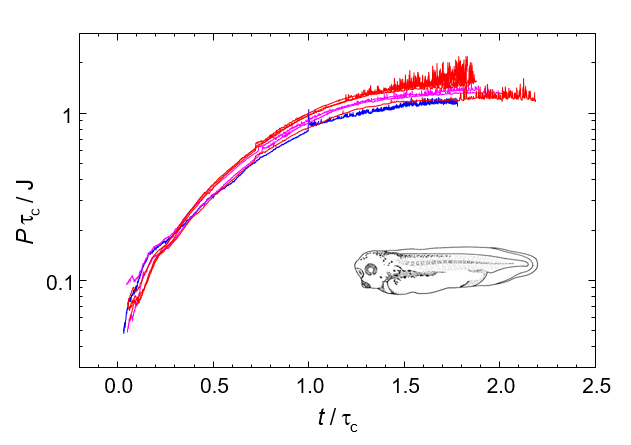
Thermal dissipations during early development of the African clawed frog, X. laevis at various temperatures. They show a complete agreement by scaling with the characterisitic time of growth, τ which depends on temperature.
High-Precision Micro-Combustion Calorimetry
The least unit of energy is roughly 1 kJ/mol for strictly describing living activities, chemical changes and chemical equilibria on the earth. High-precision combustion calorimetry provides the most effective method to determine bond energies of chemical substances in such a precision. The basic technique has been established in NBS in the US during 1930s, when petroleum industries rapidly grew. Since then, it has been improved by many researchers. At the moment, this research center significantly contributes to developing the best equipment. Although high-precision combustion calorimetry is one of the most fundamental method to determine chemical thermodynamic quantities, unfortunately, it faces a serious difficulty on its inherence in chemistry. Under current research circumstances, patient research of high-precision combustion calorimetry does not have any advantage which help carrier development for young researchers. It is emphasized that the fail to inherence of the technique would result in losing potential to critically evaluate thermodynamic quantities relating chemical bonds. It is true that all the chemical bonds are only owing to Coulombic interactions between electrons, so that bond energies may be accurately derived from the first principle calculation under Born-Oppenheimer approximation, taking relativistic effects in heavy atoms into account in near future. Anyway, inherence of high-precision combustion calorimetry should not depend only on the effort of a single researcher.
J. Chem. Thermodyn. 33, 377-387 (2001)
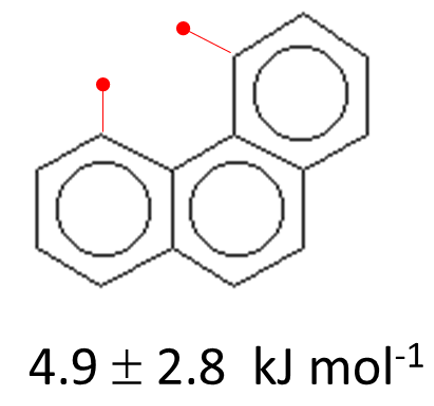
Strain energy in phenanthrene.