| Position | Professor of Institute for Advanced Co-Creation Studies at Osaka University, and Dept. of Macromolecular Science, Graduate School of Science at Osaka University | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| takasima<at>chem.sci.osaka-u.ac.jp <at> Indicates @ | |||||||||||||||||
| Career |
|
||||||||||||||||
| Degree | Ph. D from Osaka University (2003.03) | ||||||||||||||||
| Awards |
|
||||||||||||||||
| Membership in Academic ocieties | The Chemical Society of Japan, The Society of Polymer Science Japan, The Society of Cyclodextrins, Division of Biofunctional Chemistry | ||||||||||||||||
| Field of Speciakization | Macromolecular chemistry, Supramolecular Science, Metalic catalyzed polymerization |
A double-threaded dimer bearing a long substituent part and a large stopper group has been prepared and showed the conformational change with increased solvent polarity.
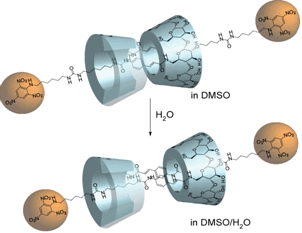
Contraction of Supramolecular Double-Threaded Dimer Formed by a-Cyclodextrin with Long Alkyl Chain.
Tsukagoshi, S.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"Contraction of Supramolecular Double-Threaded Dimer Formed by alpha-Cyclodextrin
with a Long Alkyl Chain"
Org. Lett. 2007, 9(6), 1053-1055.
We prepared a stilbene bis(b-CD) dimer and controlled its trans-cis conformation by photoirradiation in aqueous solution. With a ditopic adamantyl guest molecule, in trans conformation, it formed dimer or small supramolecular assemblies (oligomers), whereas in cis conformation, supramolecular linear polymer with high molecular weight were observed. The control of the structure of the supramolecular polymers by an external stimulus may open the way to various applications.
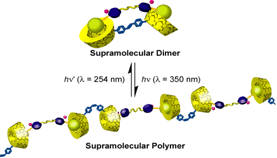
External Stimulus-Responsive Supramolecular Structures
Kuad, P.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"External Stimulus-Responsive Supramolecular Structures Formed by a Stilbene Cyclodextrin Dimer"
J. Am. Chem. Soc. 2007, 129(42), 12630-12631.
We have succeeded in controlling formation of a double threaded dimer and that of non-threaded supramolecular self-assembly consisting of stilbene amide a-CD by photoirradiation as shown in Figure 4. First, we thought that 3-trans-Sti-a-CD would form a supramolecular polymer, and then 3-cis-Sti-a-CD would decompose it after photoirradiation. The facts are contrary to what we expected. Formations of these supramolecular complexes might be due to p-p stacking interactions between stilbene amide groups. These interactions are presumed to inhibit the formation of a supramolecular polymer. Instead, it created more interesting supramolecular system, in other words, “Switching between supramolecular dimer and non-threaded supramolecular self-assembly”. The unit of a-CD and the cis-stilbene group play an important role to form supramolecular assembly as a hydrophilic and hydrophobic part, respectively. These interaction and environment promote the p-p stacking of the cis-stilbene group and affect cooperatively the formation of the supramolecular assembly.
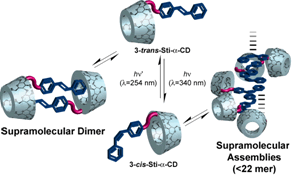
Schematic illustration of switching between supramolecular dimer and non-threaded supramolecular
self-assembly consisting of 3-Sti-a-CD with photoirradiation.
Yamauchi, K.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A.
"Switching between Supramolecular Dimer and Non-Threaded Supramolecular Self-Assembly of Stilbene Amide alpha-Cyclodextrin by Photoirradiation"
J. Am. Chem. Soc.2008, 130(15), 5024-5025.
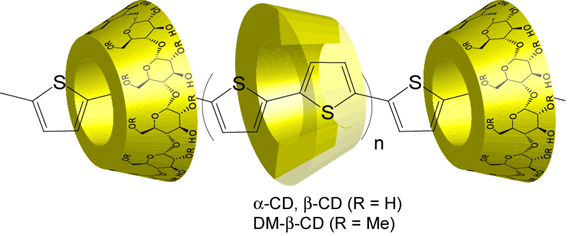
p-Conjugated polymers have attracted much interest due to their high charge-mobility along individual polymer chains. Recently, p-conjugated polymers have been used in practical applications, including light-emitting diodes, thin-film field effect transistors, photovoltaic cells, and sensors. We have prepared and determined the crystal structure of cyclodextrins inclusion complexes with various thiophenes, and the polymerization of the corresponding inclusion complexes in a selective way to obtain polyrotaxanes.
| (a) | (b) |
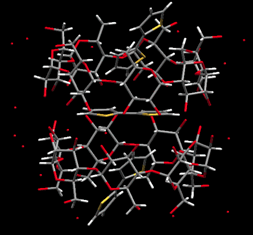 |
 |
| Crystal structure of inclusion complex. (a) 2T-2,6-O-dimethyl-β-CD (b)2T-2-β-CDβ-CD |
|
Utlizing coupling reaction, DM-β-CD poly pseudo rotaxane with polythiophene as axis was obtained.
Takashima, Y.; Sakamoto, K.; Oizumi, Y.; Yamaguchi, H.; Kamitori, S.; Harada, A.
"Complex Formation of Cyclodextrins with Various Thiophenes and Their Polymerization in Water: Preparation of pseudo-Polyrotaxanes Containing Poly(thiophene)s"
J. Incl. Phenom. Macrocycl. Chem. 2006, 56(1-2), 45-53.
Preparation and Properties of Rotaxanes Formed by Dimethyl-b-Cyclodextrin and Oligo(thiophene)s with b-Cyclodextrin Stoppers
A series of novel DM-b-CD-oligothiophene based rotaxanes have been prepared by the palladium catalyzed coupling reaction. [2]Rotaxanes and [3]rotaxanes which have various chain lengths and inclusion ratios of DM-b-CD were isolated from the crude products by using preparative reversed phase chromatography. Their photochemical properties have been investigated by UV-vis and fluorescence measurements. The inclusion ratio and the chain length of rotaxanes have been found to relate to the emission properties and the emission intensities of oligothiophene. In aqueous solutions, fluorescence quantum yields of rotaxanes were higher than those of dumbbell-shaped molecules. The increase in the fluorescence efficiency of rotaxane is caused by the suppression of intermolecular interactions, indicating the effect of insulated oligothiophene with DM-b-CD.
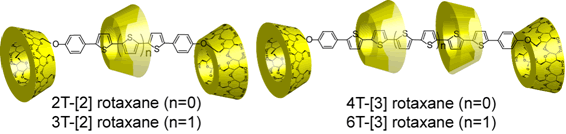
Sakamoto, K.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"Preparation and Properties of Rotaxanes Formed by Dimethyl-beta-cyclodextrin and Oligo(thiophene)s with beta-Cyclodextrin Stoppers"
J. Org. Chem. 2007, 72(2), 459-465
Cyclodextrins (CDs) have been widely employed as substrate-recognition moieties in enzyme models. CDs accelerate the hydrolysis of activated esters, such as p-nitrophenyl acetate. However, the accelerated reactions have been limited to the degradation of the activated aryl esters in the presence of excess amounts of CDs. We found that CDs selectively form inclusion complexes with some lactones (the starting materials of the polyesters) to promote or suppress the hydrolysis of lactones.
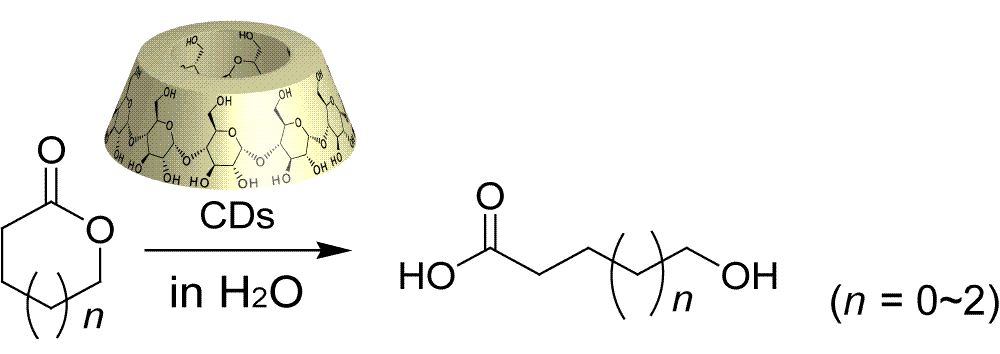
Takashima, Y.; Kawaguchi, Y.; Nakagawa, S.; Harada, A.
"Inclusion Complex Formation and Hydrolysis of Lactones by Cyclodextrins"
Chem. Lett., 2003, 32, 1122-1123.
We speculated that if lactones are heated with CDs in bulk without water, they might form polymers because hydrolysis cannot occur. CDs were found to initiate ring-opening polymerizations of lactones selectively to give polyesters in high yields in bulk without water. This polymerization system requires neither a conventional metal catalyst, nor CDs with substituent groups. The products were found to be a polymer chain attached to the hydroxyl group of CD via an ester bond.
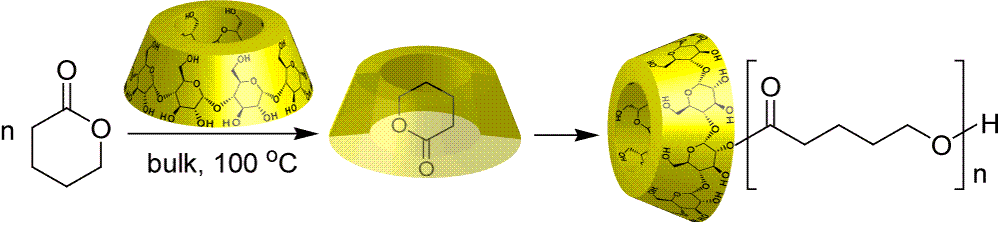
Takashima, Y.; Osaki, M.; Harada, A.
"Cyclodextrin-Initiated Polymerization of Cyclic Esters in Bulk:
Formation of Polyester-Tethered Cyclodextrins"
J. Am. Chem. Soc. 2004, 126(42), 13588-13589.
Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"Polymerization of Lactones Initiated by Cyclodextrins: Effects of Cyclodextrins on the Initiation and Propagation Reactions"
Macromolecules 2007, 40(9), 3154-3158.
Cinnamoyl CD has been found to initiate polymerization of lactone to give a polymer in high yield. The polymerization activity could be switched by the photoisomerization of the cinnamoyl group attached to the rim of CD. Specific monomer recognition and polymerization in the active site of the CD cavity is changed by the photoisomerization.
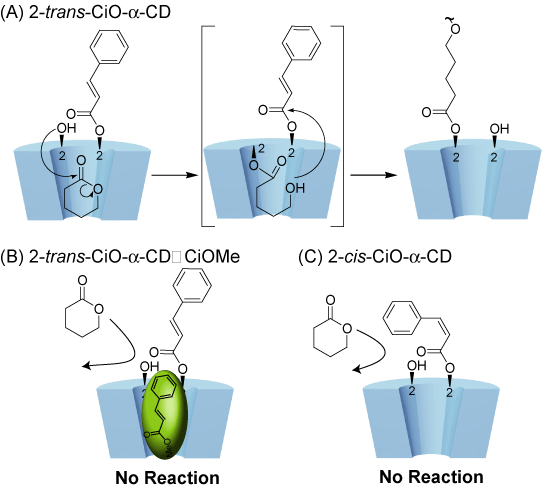
Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"Switching of Polymerization Activity of Cinnamoyl-alpha-Cyclodextrin"
Org. Biomol. Chem. 2009, 7, 1646 - 1651.
In the polymerization, the propagating polymer chain was included by the CDs. The polymer chain was found to elongate in the solid state, whereas the polymer chain without threading CDs formed random coil conformation. CDs have two roles. One CD at the end of the polymer chain initiates the polymerization. Other CDs threaded onto the polymer chain are immune to the initiation directly, but have an essential role to fold the polymer chain in a proper way as an artificial chaperone.

Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"An Artificial Molecular Chaperone: Poly-pseudo-rotaxane with an Extensible Axle"
J. Am. Chem. Soc. 2007, 129(46), 14452-14457.
Harada, A.; Osaki, M.; Takashima, Y.; Yamaguchi, H.
"Ring-Opening Polymerization of Cyclic Esters by Cyclodextrins"
Acc. Chem. Res. 2008, 41(9), 1143-1152.
CD-based nanosphere initiated the oligomerization of lactone on the surface of the nanosphere to give oligo(lactone)-tethered CD nanosphere in bulk. The addition of other CDs to the nanosphere led to the formation of poly-pseudo-rotaxane on the surface of the nanosphere. the poly-pseudo-rotaxane repropagated upon the addition of lactone. These behaviors are reminiscent of the function of a spherical virus, which forms an ordered spherical structure and releases RNA chains from the capsid surface.

Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A.
"Nanospheres with Polymerization Ability Coated by Polyrotaxane"
J. Org. Chem.2009, 74 (5), 1858-1863.
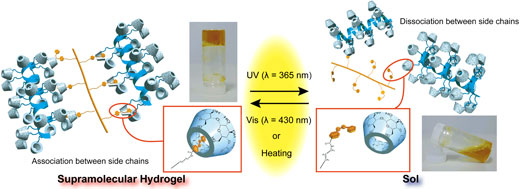
To creation of hydrogels, which was switched sol state or gel state by irradiation of UV light or Vis light, we chose the α-cyclodextrin (αCD) and the Azobenezene (Azo). The hydrogel was prepared by mixing CD modified curdlan (CD-CUR) and Azo modified polyacrylic acide (pAC12Azo), indicated that CD and Azo interacted inside the hyerogel.
To investigate effect of photo stimuli on the hydrogel, we irradiated UV and Vis to the hydrogel. When UV light irradiated to the hydrogel, the hydrogel was changed in state of sol. Subsequently, When Vis light irradiated to the hydrogel, the hydrogel was changed in state of gel.
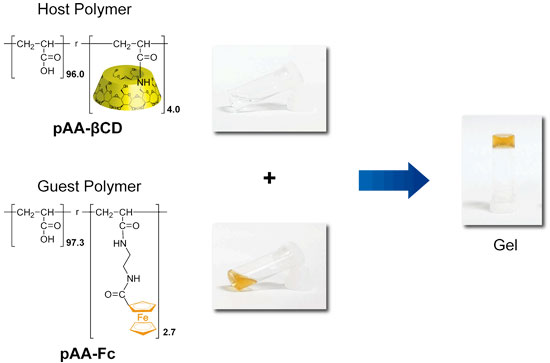
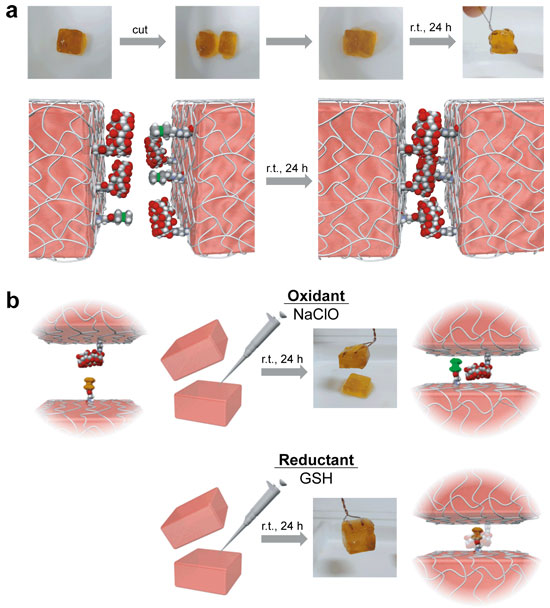
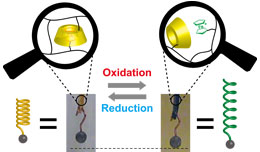
To create the redox-responsive self-healing supramolecular hydrogel, we chose the βCD as a host molecules and the ferrocene (Fc) as a guest molecules. Fc formed inclusion complexes with βCD, when Fc was reduction state. On the other hand, Fc+, which was oxidation state, dissociated inclusion complexes. The supramoleculat hydrogel was formed by mixing the Fc modified polymer and the βCD modified polymer.
 |
| Fig.1 |
The cut hydrogel pieces adhered between cut surfaces (Figure 2a).
Additionally, the self-healing performance decreased when the oxidant was coated on the cut surfaces. Subsequently, the self-healing performance recovered when the reductant was coated on the cut surfaces (Figure 2b).
 |
|
| Figure 2. The self-healing and redox-responsive behavior of the supramolecular hydrogel. | |
| (a)Readhesion experiments(b) Redox-controlled experiments of the self-healing behavior. |
|
Self-healing mechanism |
Redox-controlled experiments of the self-healing behavior |
We successfully realized reversible sol−gel switching and a self-healing supramolecular hydrogel system consisting of the β-cyclodextrin and the ferrocene.
(Nakahata, M.; Takashima, Y.; Yamaguchi H.; Harada, A., Nat. Commun. 2011, 2, 511.)
There are two basic approaches to prepare the supramolecular polymeric materials through host−guest interactions: (1) from a mixture of host and guest polymers, and (2) from polymerization of host and guest monomers. To create high strength and short self-healing materials, we chose the method of (2).
Prior to radical copolymerization, hydrophobic guest monomers (Adamantane, Ad), which are insoluble in water, were dissolved in corresponding aqueous solutions of βCDs to form inclusion complexes. After polymerization, the homogeneous solutions yielded hydrogels (Figure 1).
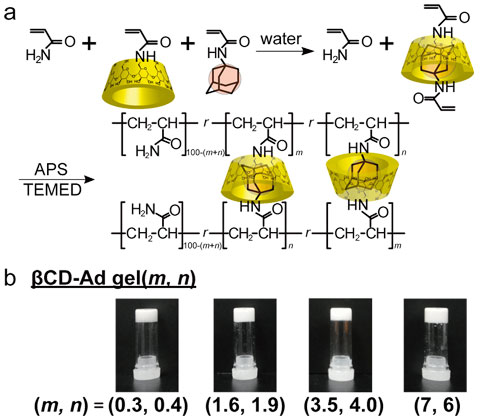
Figure 1. Chemical structures of the self-healable supramolecular hydrogel (a) and photographs of these hydrogels.
The β CDAd gel(7, 6) sample immediately mends after being broken; the gel can be lifted against its own weight. The repaired β CDAd gel(7, 6) adheres strongly without a crack after crushing and dropping (Figure 2a). 1-Adamantane carboxylic acid sodium salt (AdCANa, Figure 2 b) or β CD, in aqueous solutions, were used as the competitive molecules for the β CDAd gel(7, 6). In the presence of competitive molecules on the cut surface, the gels do not adhere within 24 h. The competitive molecules inhibit the complexation between the CD and guest units on the cut surface, which function as crosslinkers to adhere the two cut gels. We successfully prepared self-healing CDguest gels crosslinked between polymer chains with inclusion complexes. The self-healing behavior exhibited by forming inclusion complexes of the free CD and guest units on the cut surfaces (Figure 2b).
(Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A., Adv. Mater. 2013, 25, 2849.)
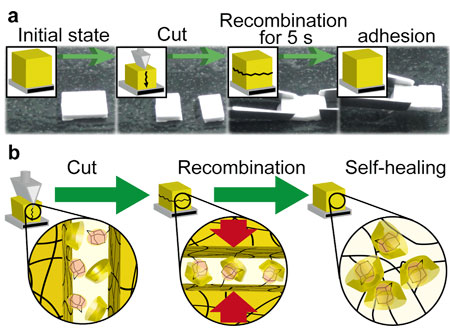 |
| Figure 2. Self-healing behavior of the host-guest gel. |
Two structural approaches may realize supramolecular actuators through host–guest interactions: a method with a linear main chain and one with a side chain in the polymer structure. Our research employs the polymer side chain method because various functions are relatively easy to be introduced into materials. Supramolecular gel are based on integrating host–guest interactions on the polymer side chains. The association and dissociation of inclusion complexes as crosslinking units on the polymer side chains demonstrate contraction and expansion motions due to changes in the crosslinking density. Topological gel is mechanically crosslinked by molecules. The drive mechanism, which involves a sliding motion in the [c2]daisy chain, is completely different from previously reported stimuli-responsive actuators, such as polymer gels, liquid crystalline elastomers, conjugated polymers, and carbon nanotubes.
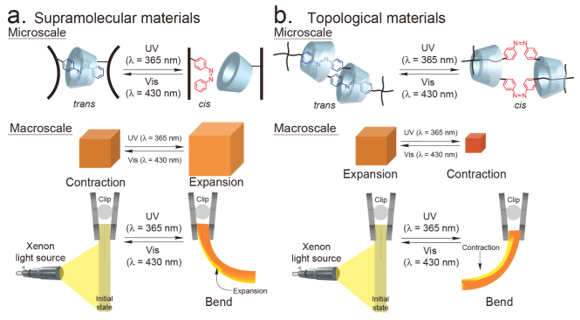
Light-Responsive Supramolecular Actuator A light-responsive artificial muscle based on the inclusion complex between α-cyclodextrin and azobenzene.
A redox-responsive supramolecular actuator based on the inclusion complex between β-cyclodextrin and ferrocene.
 |
The mechanically cross-linked hydrogel([c2]AzoCD2 hydrogel) build with four-arm poly(ethylene glycol)(tetraPEG) and [c2]daisy chains consisting of alfa-cyclodextrin(αCD) and azobenzene (Azo) showed the photo-responsive contraction and expansion behaviors. These behaviors were caused by the decrease of distance between crosslinked points related to the sliding motion of [c2]daisy chains. As a further result, the [c2]AzoCD2 xerogel lyophilized from the hydrogel have more ability to response to light than the hydrogel.
Iwaso, K.; Takashima, Y.; Harada, A. Nat. Chem. 2016, 8, 625-632.