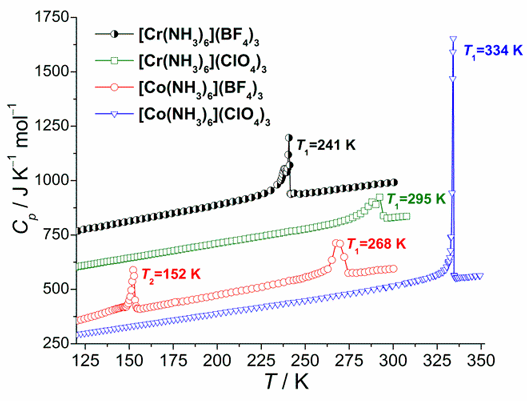
 Fig. 1. Molar heat capacity obtained for all four compounds above 120 K. All curves except for [Co(NH3)6](ClO4)3 are shifted vertically, for clarity.
Fig. 1. Molar heat capacity obtained for all four compounds above 120 K. All curves except for [Co(NH3)6](ClO4)3 are shifted vertically, for clarity.
 Fig. 1. Molar heat capacity obtained for all four compounds above 120 K. All curves except for [Co(NH3)6](ClO4)3 are shifted vertically, for clarity.
Fig. 1. Molar heat capacity obtained for all four compounds above 120 K. All curves except for [Co(NH3)6](ClO4)3 are shifted vertically, for clarity.
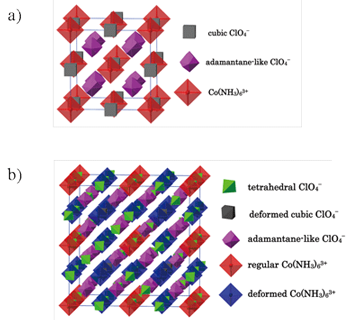 Fig. 2. Arrangement of polyhedral blocks in the unit cell of [M(NH3)6](XY4)3 on the example of [Co(NH3)6](ClO4)3 in a) the highest temperature phase I (one type of cation and two types of anion are indicated) b) lower temperature phase II (two types of cation and three types of anion are indicated).
Fig. 2. Arrangement of polyhedral blocks in the unit cell of [M(NH3)6](XY4)3 on the example of [Co(NH3)6](ClO4)3 in a) the highest temperature phase I (one type of cation and two types of anion are indicated) b) lower temperature phase II (two types of cation and three types of anion are indicated).
Phase behavior of coordination compounds of the [M(NH3)6](XY4)3 type, where M = Co3+and Cr3+, and XY4 = ClO4-and BF4-, has been investigated with adiabatic calorimetry (120 – 340 K) and X-ray single crystal diffraction (XRSCD) (200 – 340 K). From the heat capacity measurements, two phase transitions in [Co(NH3)6](BF4)3 and one phase transition in [Co(NH3)6](ClO4)3, [Cr(NH3)6](BF4)3 and [Cr(NH3)6](ClO4)3 have been discovered in solid state (see Fig. 1). All transitions obtained, except for the lower temperature one in [Co(NH3)6](BF4)3, are characterized by comparable transition entropy of the order of about 6 J K-1 mol-1.
In order to understand the mechanism of these transitions we first analyzed the crystal structure of the compounds in the highest temperature phase I (above T1). All four compounds are isostructural and crystallize in the cubic system (Fm3m space group, Z = 4). We will now describe the structure of these crystals on the example of [Co(NH3)6](ClO4)3 for better clarity. The structure consists of coordination cations [Co(NH3)6]3+ having a regular octahedral symmetry and of two types of ClO4- anions that differ in orientational disorder (see Fig. 2a). The first type of anions of cubic geometry is disordered between two possible orientations in such a way that eight half-oxygen atoms occupy the vertices of a cube with a Cl atom located at the center. The cubic anions are located at the center of the unit cell and at the middle of the edges. The anions of the second type have adamantane geometry and fill up the channels formed between sandwiched [Co(NH3)6]3+ and ClO4- groups of the first type. These anions are also disordered but number of possible orientations is difficult to estimate. There are four and eight (out of twelve) cubic and adamantane like anions, respectively, in the unit cell in phase I.
The results of X-ray single crystal diffraction measurements performed for [Co(NH3)6](ClO4)3, [Co(NH3)6](BF4)3 and [Cr(NH3)6](ClO4)3 at 200 K, thus in the lower temperature phase II, revealed that the transitions observed for these compounds are of the same nature and are a result of strong hydrogen bond like interactions between NH3 ligands and coordination anions, which occur asymmetrically in the crystal network. A view of the unit cell of phase II is shown in Fig. 2b. Three types of anions, one ordered and two dynamically disordered, can be distinguished. Namely, three-fourths of all anions of the first type and one-fourth of all anions of the second type become ordered in their orientation, as represented by tetrahedral blocks. Additionally, three-fourths of all complex cations become slightly deformed to give a lower symmetry still in a cubic system (Ia3 space group, Z = 32). These deformed cations and the ordered anions are close enough to orient some NH3 ligands, which rotate freely around their threefold symmetry axis in phase I.
Interestingly, in the highest temperature phase I all four compounds investigated are isostructural with the analogical hexammine compounds with divalent M(II) central metals, widely described in literature. However, the structure of our compounds is more compact with shorter atomic contacts, which results in drastic change in the phase behavior. The phase transitions observed in the title compounds occur at much higher temperatures, are characterized by much smaller transition entropy and are connected with different mechanism than in the compounds of the [M(NH3)6](XY4)2 type.
N. Górska, A. Inaba, Y. Hirao, and E. Mikuli, 6th International & 8th Japan-China Joint Symposium on Calorimetry and Thermal Analysis (CATS2011) (Hachiouji), 046 (2011).