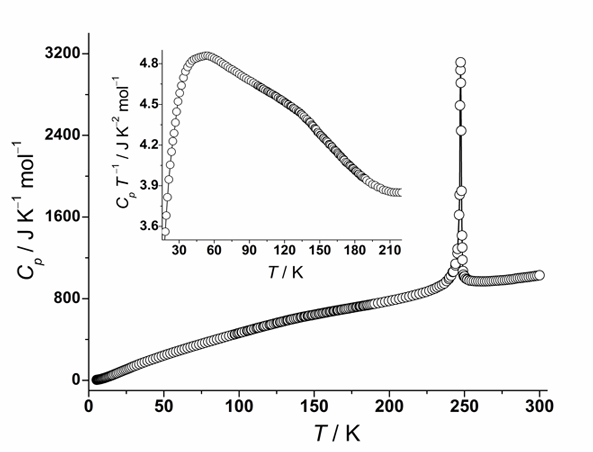
 Fig. 1. Molar heat capacity obtained for [Cr(OS(CH3)2)6](BF4)3 in the temperature range 5 – 300 K. Inset: Molar heat capacity plotted in the form of CpT-1 against T.
Fig. 1. Molar heat capacity obtained for [Cr(OS(CH3)2)6](BF4)3 in the temperature range 5 – 300 K. Inset: Molar heat capacity plotted in the form of CpT-1 against T.
 Fig. 1. Molar heat capacity obtained for [Cr(OS(CH3)2)6](BF4)3 in the temperature range 5 – 300 K. Inset: Molar heat capacity plotted in the form of CpT-1 against T.
Fig. 1. Molar heat capacity obtained for [Cr(OS(CH3)2)6](BF4)3 in the temperature range 5 – 300 K. Inset: Molar heat capacity plotted in the form of CpT-1 against T.
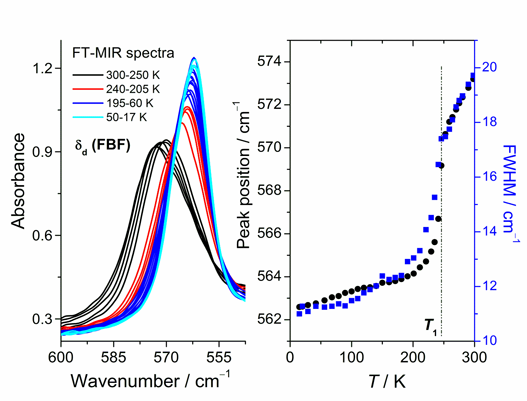 Fig. 2. Left: Temperature evolution of the FT-IR band connected with δd(FBF) vibration at 573 cm-1. Right: Temperature dependences of peak position and FWHM of this band.
Fig. 2. Left: Temperature evolution of the FT-IR band connected with δd(FBF) vibration at 573 cm-1. Right: Temperature dependences of peak position and FWHM of this band.
In our previous study we investigated the solid polymorphism of [Cr(NH3)6](BF4)3. We discovered one phase transition at T1 = 241 K connected with ordering of some BF4− anions together with distortion of [Cr(NH3)6]3+ complex cations due to hydrogen bond interactions and two phase transitions at T2 = 108 K and T3 = 61 K most probably connected with further ordering of the rest of disordered anions. It was interesting to analyze how the replacement of ligand would affect the phase behavior of this type of compound. For this purpose, [Cr(OS(CH3)2)6](BF4)3 has been synthesized and studied by means of adiabatic calorimetry in the temperatures between 5 and 300 K. Two phase transitions have been discovered, one very sharp and distinct at T1 = 247 K and one very broad extending between 60 and 180 K with the maximum at T2 ~ 135 K (see Fig. 1). Interestingly, the entropy change of the high temperature transition is equal to 26.2 J K-1 mol-1, which is close to the value of ΔS = 3 R ln 3 = 27.4 J
K-1 mol-1. It can suggest that all BF4− anions are dynamically disordered and perform fast reorientational motion between three possible orientations in the highest temperature phase and that below the transition at T1 this reorientation is suddenly stopped.
The observed phase transitions have been also studied using middle infrared absorption spectroscopy (FT-MIR) in the temperatures between 17 and 300 K. The drastic changes in the bands positions and intensities connected with the vibrations within OS(CH3)2 groups as well as BF4- anions have been observed around the transition at T1. Fig. 2 presents temperature evolution of the band connected with δd(FBF) vibration together with the analyses of peak position and full width at half maximum (FWHM) of this band. The behaviors of both investigated quantities are basically the same. In the first place, a large shift of the peak position toward lower frequency and large reduction of FWHM value can be observed in the phase transition region at T1 and down to about 200 K. Next, below this temperature the peak position and FWHM values become nearly constant. This behavior implies that the phase transition observed in [Cr(DMSO)6](BF4)3 at T1 is indeed connected with rapid change in the speed of reorientational motions of the BF4- groups. The spectral width of this band yielded an activation energy Ea = 8.0 kJ mol-1, which is comparable to the values obtained for other complexes with BF4- anions. Fig. 3 presents temperature evolution of the band connected with ρr(CH3) vibration together with the analysis of peak position of this band. Contrary to the previous observation, distinct shift of the band position toward higher frequency is observed at T1 with decreasing temperature. It suggests that OS(CH3)2---BF hydrogen bond like interactions can play important role in the transition at T1.
Although the transition at T2 is not pronounced in the heat capacity curve, the changes in FT-MIR spectra could be also observed in this region. Namely, splitting of most bands connected with the CH3, SO, CS and CrO vibrations into three components in the lowest temperature phase (below T2) is most probably connected with lowering of the symmetry of [Cr(OS(CH3)2)6]3+ complex cation and OS(CH3)2 ligands.
It should be also pointed out that the phase behavior of the titled compound differs significantly from the one observed in divalent analogs of the [M(DMSO)6](BF4)2 type, which undergo phase transitions between stable and metastable phases mainly above room temperature.
N. Górska, A. Inaba, and A. Migdał-Mikuli, 6th International & 8th Japan-China Joint Symposium on Calorimetry and Thermal Analysis (CATS2011) (Hachiouji), PA14 (2011).
Copyright © Research Center for Structural Thermodynamics, Graduate School of Science, Osaka University. All rights reserved.