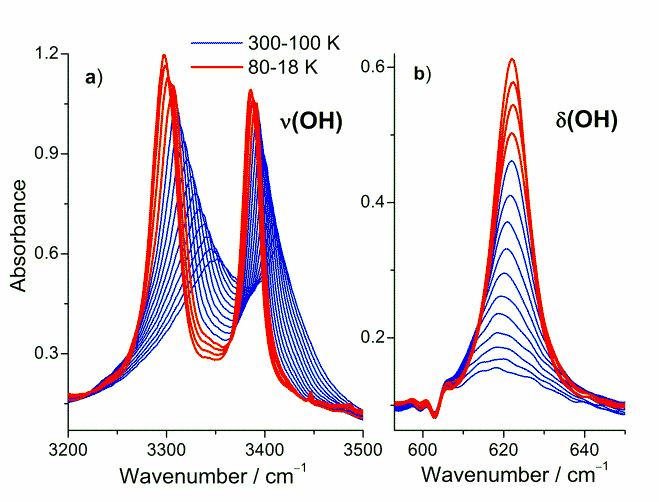
 Fig. 1. Temperature evolutions of the FT-IR bands connected with ν(OH) and δ(OH) vibrations.
Fig. 1. Temperature evolutions of the FT-IR bands connected with ν(OH) and δ(OH) vibrations.When diacetylene derivatives are exposed to ultraviolet light or high energy in the solid phase they can easily undergo polymerization reaction giving polymers with conjugated double and triple bonds. Such polymers exhibit number of interesting and promising features such as nonlinear optical effects or electronic conductivity, which can be used in optical and electronic devices. 10,12-pentacosadiyn-1-ol [CH3(CH2)11C≡C−C≡C(CH2)9OH] is one of the linear diacetylenes, which has a hydrophilic and a hydrophobic group in a molecule and can be used to obtain ultrathin photosensitive films with Langmuir-Blodgett (LB) technique.
It seemed essential to investigate an unpolymerized crystalline material in order to understand the formation of mono- or multilayer systems and for their proper characterization. Thermal behavior of 10,12-pentacosadiyn-1-ol has been already investigated using adiabatic calorimetry [this report No. 30 (2009) #8]. In the temperature range of 5 – 300 K the compound undergoes one solid-solid phase transition at T1 = 90.4 K. The transition observed was not accompanied by latent heat. Also, the transition entropy value was estimated to be 2.8 J K-1 mol-1, which is close to ½ R ln 2, suggesting the second-order type of this transition.
In this study, further analysis of this transition by means of Fourier transform middle infrared absorption spectroscopy (FT-MIR) has been performed in temperatures between 18 and 300 K. The FT-IR spectra were obtained for polycrystalline sample suspended in nujol and measured during cooling, in frequency range of 400 – 4000 cm-1.
 Fig. 1. Temperature evolutions of the FT-IR bands connected with ν(OH) and δ(OH) vibrations.
Fig. 1. Temperature evolutions of the FT-IR bands connected with ν(OH) and δ(OH) vibrations.
Fig. 1a presents the temperature evolution of the bands located at 3351 and 3414 cm-1 (at 300 K) connected with ν(OH) vibrations. The location of these bands is characteristic for polymeric material in which long chains of molecules are linked by weak hydrogen bonds without dimeric association or free OH groups from monomeric molecules. The shift of the bands position toward lower frequency and the large increase of intensity were observed with decreasing temperature. The bands at lower temperatures become very sharp and almost separated. The large increase in intensity with decreasing temperature is also visible for the band at 620 cm-1 which is connected with δ(OH) vibration (out of plane bending) (Fig. 1b). Temperature behavior of both bands indicate that the OH groups are involved in hydrogen bonding and their bond strength increases with decreasing temperature but there is no abrupt change across the phase transition.
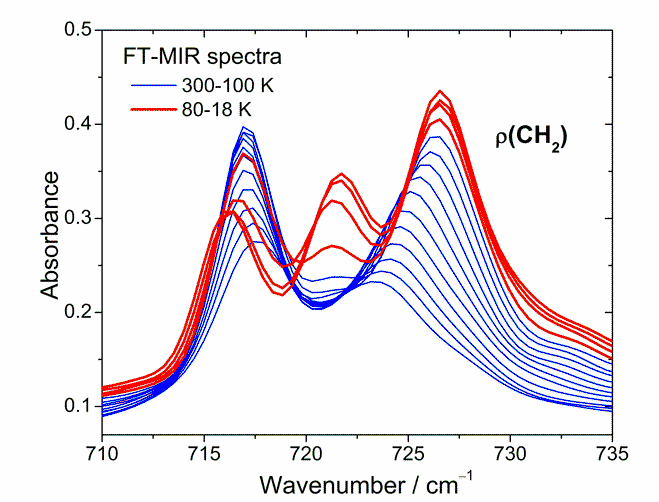 Fig. 2. Temperature evolution of the FT-IR bands connected with ρ(CH2) vibration observed in the compounds with long aliphatic chain.
Fig. 2. Temperature evolution of the FT-IR bands connected with ρ(CH2) vibration observed in the compounds with long aliphatic chain.
The other gradual dynamic changes like significant increase in intensities of the bands connected with δ(CH2), ν(CO) and ν(C≡C) vibrations together with concurrent shifting of the band positions connected with ν(CO) vibrations toward higher frequency were also observed with decreasing temperature. All these changes occur also without sudden change in behavior except for one region. Fig. 2 presents temperature evolution of the FT-IR bands in the spectral range between 710 and 735 cm-1 connected with ρ(CH2) vibrations, which are indicative of a long-chain aliphatic structure. Intensities of the two bands visible at 718 and 723 cm-1 (at 300 K) increase gradually with decreasing temperature down to about 100 K. Below this temperature, thus in the phase transition region intensity of the lower frequency band suddenly decreases together with appearance of a new band at about 722 cm-1. This behavior suggests that the aliphatic chain is involved in the transition. Perhaps the stronger hydrogen bond interactions being created at lower temperature cause the aliphatic chain to slightly fold.
Taking into consideration the FT-IR results it can be concluded that the transition observed at T1 is not connected with a significant change in a crystal structure but is rather of very moderate dynamic nature. Single crystal investigations remain crucial for understanding the mechanism of the transition.
Copyright © Research Center for Structural Thermodynamics, Graduate School of Science, Osaka University. All rights reserved.