An ion-responsive gel sticking to a target surface
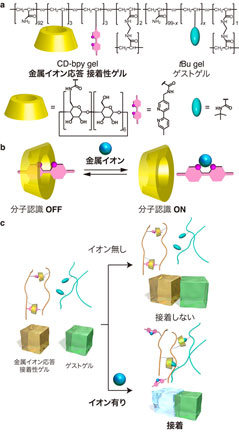
We developed a polymer hydrogel, CDbpy gel. The gel was modified with both cyclodextrin (CD), a cyclic oligosaccharide with molecular recognition ability, and 2,2´-bipyridyl (bpy), a metal-ion-responsive unit.
The CD-bpy gel exhibited surface-selective adhesion property based on molecular recognition by CD, only in the presence of metal ions. Furthermore, adhesion property can be controlled by the kinds of metal ions, depending on the efficiency of metal-bpy complexes in cross-linking. This resul
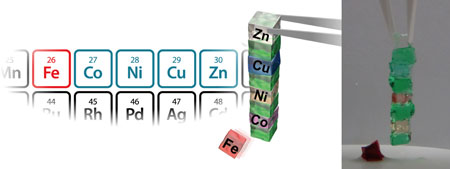
t is the world’s first achievement that controlled chemically-selective adhesion based on host-guest interactions by metal ions as independent chemical stimuli.
Nakamura, T.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A., Nature Communications 2014,5, 4622.
(DOI: 10.1038/ncomms5622)
Return to Top

 t is the world’s first achievement that controlled chemically-selective adhesion based on host-guest interactions by metal ions as independent chemical stimuli.
t is the world’s first achievement that controlled chemically-selective adhesion based on host-guest interactions by metal ions as independent chemical stimuli.